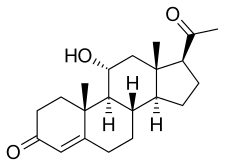
11α-Hydroxyprogesterone
 | |
| Clinical data | |
|---|---|
| Other names | 11α-OHP; 11α-Hydroxypregn-4-ene-3,20-dione; 4-Pregnen-11α-ol-3,20-dione; δ4-Pregnen-11α-ol-3,20-dione |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.001.189 |
| Chemical and physical data | |
| Formula | C21H30O3 |
| Molar mass | 330.468 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
11α-Hydroxyprogesterone (11α-OHP), or 11α-hydroxypregn-4-ene-3,20-dione is an endogenous steroid and metabolite of progesterone.[1][2][3] It is a weak antiandrogen, and is devoid of androgenic, estrogenic, and progestogenic activity.[4][5][6] It was investigated as a topical antiandrogen for the treatment of androgen-dependent skin conditions in the early 1950s, and was found to produce some benefit.[7] In 1995, 11α-OHP, along with its epimer 11β-hydroxyprogesterone, was identified as a very potent competitive inhibitor of both isoforms (1 and 2) of 11β-hydroxysteroid dehydrogenase (11β-HSD).[2][3] It is notably not metabolized by 11β-HSD2.[8] 11α-OHP is a more potent inhibitor of 11β-HSD than enoxolone (glycyrrhetinic acid) or carbenoxolone in vitro (IC50 = 0.9 nM; IC50 = 5 nM in transfected cells).[8][9][10] The compound has been found to be highly active in conferring mineralocorticoid sodium-retaining activity of corticosterone in vivo in rat bioassays and in increasing blood pressure, effects that it mediates by preventing the 11β-HSD-mediated inactivation of endogenous corticosteroids.[2][3] Because of its inhibition of 11β-HSD and consequent potentiation of corticosteroids, 11α-OHP has recently been patented for the treatment of skin diseases, particularly psoriasis in combination with clobetasol propionate and minoxidil.[5]
11α-OHP is used as a precursor in chemical syntheses of cortisone and hydrocortisone.[11][12][13]
See also
- Steroidal antiandrogen
- List of steroidal antiandrogens
- Glycyrrhizin
References
- ↑ Ford, Donald H. (1954). "EFFECT OF 11α-HYDROXYPROGESTERONE ON REPRODUCTIVE SYSTEM OF NORMAL AND PREGNANT ADULT WISTAR RATS*". The Journal of Clinical Endocrinology & Metabolism. 14 (10): 1268–1270. doi:10.1210/jcem-14-10-1268. ISSN 0021-972X. PMID 13201630.
- 1 2 3 Souness GW, Latif SA, Laurenzo JL, Morris DJ (1995). "11 alpha- and 11 beta-hydroxyprogesterone, potent inhibitors of 11 beta-hydroxysteroid dehydrogenase (isoforms 1 and 2), confer marked mineralocorticoid activity on corticosterone in the ADX rat". Endocrinology. 136 (4): 1809–12. doi:10.1210/endo.136.4.7895695. PMID 7895695.
- 1 2 3 Souness GW, Morris DJ (1996). "11 alpha- and 11 beta-hydroxyprogesterone, potent inhibitors of 11 beta-hydroxysteroid dehydrogenase, possess hypertensinogenic activity in the rat". Hypertension. 27 (3 Pt 1): 421–5. doi:10.1161/01.hyp.27.3.421. PMID 8698448.
- ↑ Lerner, Leonard J. (1975). "Androgen antagonists". Pharmacology & Therapeutics B. 1 (2): 217–231. doi:10.1016/0306-039X(75)90006-9. ISSN 0306-039X. PMID 772705.
11α Hydroxyprogesterone, while devoid of androgenic, estrogenic and progestational activity, is weakly anti androgenic in castrate rats.
- 1 2 Nguyen, Kim Thoa; Virus, Cornelia; Günnewich, Nils; Hannemann, Frank; Bernhardt, Rita (2012). "Changing the Regioselectivity of a P450 from C15 to C11 Hydroxylation of Progesterone". ChemBioChem. 13 (8): 1161–1166. doi:10.1002/cbic.201100811. ISSN 1439-4227. PMID 22532270. S2CID 34483686.
11α-Hydroxyprogesterone is an important pharmaceutical compound with anti-androgenic and blood-pressure-regulating activity. [...] 11α-Hydroxyprogesterone can therefore influence blood pressure regulation.12 Furthermore, 11α-hydroxyprogesterone exhibits an anti-androgenic activity with minimal estrogenic and progestational side effects.13 This substance was also recently patented for its role in treating skin diseases, especially for psoriasis in combination with clobetasol propionate and minoxidil.14.
- ↑ Tindall, D.J.; Chang, C.H.; Lobl, T.J.; Cunningham, G.R. (1984). "Androgen antagonists in androgen target tissues". Pharmacology & Therapeutics. 24 (3): 367–400. doi:10.1016/0163-7258(84)90010-X. ISSN 0163-7258. PMID 6205409.
- ↑ Larry E. Millikan (19 April 2016). Drug Therapy in Dermatology. CRC Press. p. 403. ISBN 978-0-203-90831-0.
Topical antiandrogens have also been tried, including topical progesterone, which proved ineffective. However, small studies with topical 11α-hydroxyprogesterone and 17α-estradiol showed some benefit [38,39].
- 1 2 Morita, H; Zhou, M; Foecking, M F; Gomez-Sanchez, E P; Cozza, E N; Gomez-Sanchez, C E (1996). "11 beta-Hydroxysteroid dehydrogenase type 2 complementary deoxyribonucleic acid stably transfected into Chinese hamster ovary cells: specific inhibition by 11 alpha-hydroxyprogesterone". Endocrinology. 137 (6): 2308–2314. doi:10.1210/endo.137.6.8641180. ISSN 0013-7227. PMID 8641180.
11 alpha-Hydroxyprogesterone (11 alpha OH-P) was an order of magnitude more potent a competitive inhibitor of the 11 beta HSD-2 than was glycyrrhetinic acid (GA) (approximate IC50 = 0.9 vs. 15 nM).
- ↑ Tomlinson, Jeremy W.; Walker, Elizabeth A.; Bujalska, Iwona J.; Draper, Nicole; Lavery, Gareth G.; Cooper, Mark S.; Hewison, Martin; Stewart, Paul M. (2004). "11β-Hydroxysteroid Dehydrogenase Type 1: A Tissue-Specific Regulator of Glucocorticoid Response". Endocrine Reviews. 25 (5): 831–866. doi:10.1210/er.2003-0031. ISSN 0163-769X. PMID 15466942.
In intact cells 11α-hydroxyprogesterone is a more potent inhibitor of 11β-HSD1 than glycyrrhetinic acid or 11β-hydroxyprogesterone (117, 118).
- ↑ Bujalska, Iwona; Shimojo, Masako; Howie, Alex; Stewart, Paul M. (1997). "Human 11β-hydroxysteroid dehydrogenase: Studies on the stably transfected isoforms and localization of the type 2 isozyme within renal tissue". Steroids. 62 (1): 77–82. doi:10.1016/S0039-128X(96)00163-8. ISSN 0039-128X. PMID 9029719. S2CID 22551136.
- ↑ Peter J. Dunn; Andrew Wells; Michael T. Williams (2 February 2010). Green Chemistry in the Pharmaceutical Industry. John Wiley & Sons. pp. 2–. ISBN 978-3-527-62969-5.
- ↑ Clemens Lamberth; Jürgen Dinges (17 May 2016). Bioactive Carboxylic Compound Classes: Pharmaceuticals and Agrochemicals. Wiley. pp. 250–. ISBN 978-3-527-69396-2.
- ↑ Kishan Gopal Ramawat; Jean-Michel Mérillon (16 October 2008). Bioactive Molecules and Medicinal Plants. Springer Science & Business Media. pp. 5–. ISBN 978-3-540-74603-4.