Streptococcus pyogenes
| Streptococcus pyogenes | |
|---|---|
_(52602981880).jpg.webp) | |
| False-color scanning electron micrograph of chain of Streptococcus pyogenes bacteria (yellow) | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Bacillota |
| Class: | Bacilli |
| Order: | Lactobacillales |
| Family: | Streptococcaceae |
| Genus: | Streptococcus |
| Species: | S. pyogenes |
| Binomial name | |
| Streptococcus pyogenes Rosenbach 1884 | |
Streptococcus pyogenes is a species of Gram-positive, aerotolerant bacteria in the genus Streptococcus. These bacteria are extracellular, and made up of non-motile and non-sporing cocci (round cells) that tend to link in chains. They are clinically important for humans, as they are an infrequent, but usually pathogenic, part of the skin microbiota that can cause Group A streptococcal infection. S. pyogenes is the predominant species harboring the Lancefield group A antigen, and is often called group A Streptococcus (GAS). However, both Streptococcus dysgalactiae and the Streptococcus anginosus group can possess group A antigen as well. Group A streptococci, when grown on blood agar, typically produce small (2–3 mm) zones of beta-hemolysis, a complete destruction of red blood cells. The name group A (beta-hemolytic) Streptococcus (GABHS) is thus also used.[1]
The species name is derived from Greek words meaning 'a chain' (streptos) of berries (coccus [Latinized from kokkos]) and pus (pyo)-forming (genes), since a number of infections caused by the bacterium produce pus. The main criterion for differentiation between Staphylococcus spp. and Streptococcus spp. is the catalase test. Staphylococci are catalase positive whereas streptococci are catalase-negative.[2] S. pyogenes can be cultured on fresh blood agar plates. The PYR test allows for the differentiation of Streptococcus pyogenes other morphologically similar beta hemolytic streptococci ( such as S. dysgalactiae subsp. esquismilis) as S. pyogenes will produce a positive test result.[3] Under ideal conditions, it has an incubation period of 1 to 3 days.[4]
An estimated 700 million GAS infections occur worldwide each year. While the overall mortality rate for these infections is less than 0.1%, over 650,000 of the cases are severe and invasive, with these cases having a mortality rate of 25%.[5] Early recognition and treatment are critical; diagnostic failure can result in sepsis and death.[6][7]
Epidemiology

S. pyogenes typically colonizes the throat, genital mucosa, rectum, and skin. Of healthy individuals, 1% to 5% have throat, vaginal, or rectal carriage. In healthy children, such carriage rate varies from 2 to 17%. There are four methods for the transmission of this bacterium: inhalation of respiratory droplets, skin contact, contact with objects, surface, or dust that is contaminated with bacteria or, less commonly, transmission through food. Such bacteria can cause a variety of diseases such as streptococcal pharyngitis, rheumatic fever, rheumatic heart disease, and scarlet fever. Although pharyngitis is mostly viral in origin, about 15 to 30% of all pharyngitis cases in children are caused by GAS; meanwhile, 5 to 20% of pharyngitis in adults are streptococcal. The number of pharyngitis cases is higher in children than in adults due to exposures in schools, nurseries, and as a consequence of lower host immunity. Cases of Streptococcal pharyngitis occur more frequently in later winter to early spring in seasonal countries due to many people rebreathing the same indoor air. Disease cases are the lowest during autumn.[8]
The MT1 (metabolic type 1) clone is frequently associated with invasive Streptococcus pyogenes infections among developed countries. The incidence and mortality of S. pyogenes was high during the pre-penicillin era, but had already started to fall prior to the widespread availability of penicillin. Therefore, environmental factors do play a role in the S. pyogenes infection. Incidence of S. pyogenes is 2 to 4 per 100,000 population in developed countries and 12 to 83 per 100,000 population in developing countries. S. pyogenes infection is more frequently found in men than women, with highest rates in the elderly, followed by infants. In people with risk factors such as heart disease, diabetes, malignancy, blunt trauma, surgical incision, virus respiratory infection, including influenza, S. pyogenes infection happens in 17 to 25% of all cases. GAS secondary infection usually happens within one week of the diagnosis of influenza infection. In 14 to 16% of childhood S. pyogenes infections, there is a prior chickenpox infection. Such S. pyogenes infection in children usually manifests as severe soft tissue infection with onset 4 to 12 days from the chickenpox diagnosis. There is also 40 to 60 times increase in risk of S. pyogenes infection within the first two weeks of chickenpox infection in children. However, 20 to 30% of S. pyogenes infection does occur in adults with no identifiable risk factors. The incidence is higher in children (50 to 80% of S. pyogenes infection) with no known risk factors. The rates of scarlet fever in UK was usually 4 in 100,000 population, however, in 2014, the rates had risen to 49 per 100,000 population. Rheumatic fever and rheumatic heart disease (RHD) usually occurs at 2 to 3 weeks after the throat infection, which is more common among the impoverished people in developing countries. From 1967 to 1996, the global mean incidence of rheumatic fever and RHD was 19 per 100,000 with the highest incidence at 51 per 100,000.[8]
Maternal S. pyogenes infection usually happens in late pregnancy; at more than 30 weeks of gestation to four weeks postpartum, which accounts for 2 to 4% of all the S. pyogenes infections. This represents 20 to 100 times increase in risk for S. pyogenes infections. Clinical manifestations are: pneumonia, septic arthritis, necrotizing fasciitis, and genital tract sepsis. According to a study done by Queen Charlotte's hospital in London during the 1930s, the vagina was not the common source of such infection. On the contrary, maternal throat infection and close contacts with carriers were the more common sites for maternal S. pyogenes infection.[8]
Bacteriology
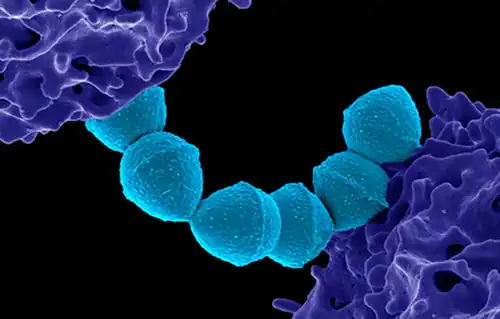 Colorized scanning electron micrograph of Group A Streptococcus (Streptococcus pyogenes) bacteria blue and a human neutrophil
Colorized scanning electron micrograph of Group A Streptococcus (Streptococcus pyogenes) bacteria blue and a human neutrophil_(52606801786).jpg.webp) False-color scanning electron microscope image of Streptococcus pyogenes (orange) during phagocytosis with a human neutrophil
False-color scanning electron microscope image of Streptococcus pyogenes (orange) during phagocytosis with a human neutrophil
Serotyping
In 1928, Rebecca Lancefield published a method for serotyping S. pyogenes based on its cell-wall polysaccharide,[9] a virulence factor displayed on its surface.[10] Later, in 1946, Lancefield described the serologic classification of S. pyogenes isolates based on their surface T-antigen.[11] Four of the 20 T-antigens have been revealed to be pili, which are used by bacteria to attach to host cells.[12] As of 2016, a total of 120 M proteins are identified. These M proteins are encoded by 234 types emm gene with greater than 1,200 alleles.[8]
Lysogeny
All strains of S. pyogenes are polylysogenized, in that they carry one or more bacteriophage on their genomes.[13] Some of the 'phages may be defective, but in some cases active 'phage may compensate for defects in others.[14] In general, the genome of S. pyogenes strains isolated during disease are >90% identical, they differ by the 'phage they carry.[15]
Virulence factors
S. pyogenes has several virulence factors that enable it to attach to host tissues, evade the immune response, and spread by penetrating host tissue layers.[16] A carbohydrate-based bacterial capsule composed of hyaluronic acid surrounds the bacterium, protecting it from phagocytosis by neutrophils.[2] In addition, the capsule and several factors embedded in the cell wall, including M protein, lipoteichoic acid, and protein F (SfbI) facilitate attachment to various host cells.[17] M protein also inhibits opsonization by the alternative complement pathway by binding to host complement regulators. The M protein found on some serotypes is also able to prevent opsonization by binding to fibrinogen.[2] However, the M protein is also the weakest point in this pathogen's defense, as antibodies produced by the immune system against M protein target the bacteria for engulfment by phagocytes. M proteins are unique to each strain, and identification can be used clinically to confirm the strain causing an infection.[18]
| Name | Description |
|---|---|
| Streptolysin O | An exotoxin, one of the bases of the organism's beta-hemolytic property, streptolysin O causes an immune response and detection of antibodies to it; antistreptolysin O (ASO) can be clinically used to confirm a recent infection. It is damaged by oxygen. |
| Streptolysin S | A cardiotoxic exotoxin, another beta-hemolytic component, not immunogenic and O2 stable: A potent cell poison affecting many types of cell including neutrophils, platelets, and subcellular organelles. |
| Streptococcal pyrogenic exotoxin A (SpeA) | Superantigens secreted by many strains of S. pyogenes: This pyrogenic exotoxin is responsible for the rash of scarlet fever and many of the symptoms of streptococcal toxic shock syndrome, also known as toxic shock like syndrome (TSLS). |
| Streptococcal pyrogenic exotoxin C (SpeC) | |
| Streptococcal pyrogenic exotoxin B (SpeB) | A cysteine protease and the predominant secreted protein. Multiple actions, including degrading the extracellular matrix, cytokines, complement components, and immunoglobulins. Also called streptopain.[19] |
| Streptokinase | Enzymatically activates plasminogen, a proteolytic enzyme, into plasmin, which in turn digests fibrin and other proteins |
| Hyaluronidase | Hyaluronidase is widely assumed to facilitate the spread of the bacteria through tissues by breaking down hyaluronic acid, an important component of connective tissue. However, very few isolates of S. pyogenes are capable of secreting active hyaluronidase due to mutations in the gene that encodes the enzyme. Moreover, the few isolates capable of secreting hyaluronidase do not appear to need it to spread through tissues or to cause skin lesions.[20] Thus, the true role of hyaluronidase in pathogenesis, if any, remains unknown. |
| Streptodornase | Most strains of S. pyogenes secrete up to four different DNases, which are sometimes called streptodornase. The DNases protect the bacteria from being trapped in neutrophil extracellular traps (NETs) by digesting the NETs' web of DNA, to which are bound neutrophil serine proteases that can kill the bacteria.[21] |
| C5a peptidase | C5a peptidase cleaves a potent neutrophil chemotaxin called C5a, which is produced by the complement system.[22] C5a peptidase is necessary to minimize the influx of neutrophils early in infection as the bacteria are attempting to colonize the host's tissue.[23] C5a peptidase, although required to degrade the neutrophil chemotaxin C5a in the early stages of infection, is not required for S. pyogenes to prevent the influx of neutrophils as the bacteria spread through the fascia.[24] |
| Streptococcal chemokine protease | The affected tissue of patients with severe cases of necrotizing fasciitis are devoid of neutrophils.[25] The serine protease ScpC, which is released by S. pyogenes, is responsible for preventing the migration of neutrophils to the spreading infection. ScpC degrades the chemokine IL-8, which would otherwise attract neutrophils to the site of infection.[23][24] |
Genome
The genomes of different strains were sequenced (genome size is 1.8–1.9 Mbp)[26] encoding about 1700-1900 proteins (1700 in strain NZ131,[27][28] 1865 in strain MGAS5005[29][30]). Complete genome sequences of the type strain of S. pyogenes (NCTC 8198T Archived 2021-12-17 at the Wayback Machine = CCUG 4207T Archived 2023-01-13 at the Wayback Machine) are available in DNA Data Bank of Japan, European Nucleotide Archive, and GenBank under the accession numbers LN831034 Archived 2023-01-13 at the Wayback Machine and CP028841 Archived 2023-01-13 at the Wayback Machine.[31]
Biofilm formation
Biofilms are a way for S. pyogenes, as well as other bacterial cells, to communicate with each other. In the biofilm gene expression for multiple purposes (such as defending against the host immune system) is controlled via quorum sensing.[32] One of the biofilm forming pathways in GAS is the Rgg2/3 pathway. It regulates SHP's (short hydrophobic peptides) that are quorum sensing pheromones a.k.a. autoinducers. The SHP's are translated to an immature form of the pheromone and must undergo processing, first by a metalloprotease enzyme inside the cell and then in the extracellular space, to reach their mature active form. The mode of transportation out of the cell and the extracellular processing factor(s) are still unknown. The mature SHP pheromone can then be taken into nearby cells and the cell it originated from via a transmembrane protein, oligopeptide permease.[32] In the cytosol the pheromones have two functions in the Rgg2/3 pathway. Firstly, they inhibit the activity of Rgg3 which is a transcriptional regulator repressing SHP production. Secondly, they bind another transcriptional regulator, Rgg2, that increases the production of SHP's, having an antagonistic effect to Rgg3. SHP's activating their own transcriptional activator creates a positive feedback loop, which is common for the production for quorum sensing peptides. It enables the rapid production of the pheromones in large quantities. The production of SHP's increases biofilm biogenesis.[32] It has been suggested that GAS switches between biofilm formation and degradation by utilizing pathways with opposing effects. Whilst Rgg2/3 pathway increases biofilm, the RopB pathway disrupts it. RopB is another Rgg-like protein (Rgg1) that directly activates SpeB (Streptococcal pyrogenic exotoxin B), a cysteine protease that acts as a virulence factor. In the absence of this pathway, biofilm formation is enhanced, possibly due to the lack of the protease degrading pheromones or other Rgg2/3 pathway counteracting effects.[32]
Disease
S. pyogenes is the cause of many human diseases, ranging from mild superficial skin infections to life-threatening systemic diseases.[2] Infections typically begin in the throat or skin. The most striking sign is a strawberry-like rash. Examples of mild S. pyogenes infections include pharyngitis (strep throat) and localized skin infection (impetigo). Erysipelas and cellulitis are characterized by multiplication and lateral spread of S. pyogenes in deep layers of the skin. S. pyogenes invasion and multiplication in the fascia can lead to necrotizing fasciitis, a life-threatening condition which requires prompt surgical intervention to reduce morbidity and mortality.[33][34] The bacterium is found in neonatal infections.[35]
Infections due to certain strains of S. pyogenes can be associated with the release of bacterial toxins. Throat infections associated with release of certain toxins lead to scarlet fever. Other toxigenic S. pyogenes infections may lead to streptococcal toxic shock syndrome, which can be life-threatening.[2]
S. pyogenes can also cause disease in the form of post-infectious "non-pyogenic" (not associated with local bacterial multiplication and pus formation) syndromes. These autoimmune-mediated complications follow a small percentage of infections and include rheumatic fever and acute post-infectious glomerulonephritis. Both conditions appear several weeks following the initial streptococcal infection. Rheumatic fever is characterized by inflammation of the joints and/or heart following an episode of streptococcal pharyngitis. Acute glomerulonephritis, inflammation of the renal glomerulus, can follow streptococcal pharyngitis or skin infection.
This bacterium remains acutely sensitive to penicillin. Failure of treatment with penicillin is generally attributed to other local commensal organisms producing β-lactamase, or failure to achieve adequate tissue levels in the pharynx. Certain strains have developed resistance to macrolides, tetracyclines, and clindamycin.
Vaccine
There is a polyvalent inactivated vaccine against several types of Streptococcus including S. pyogenes called " vacuna antipiogena polivalente BIOL" it is recommended an administration in a series of 5 weeks. Two weekly applications are made at intervals of 2 to 4 days. The vaccine is produced by the Instituto Biológico Argentino.[36]
There is another potential vaccine being developed; the vaccine candidate peptide is called StreptInCor.[37]
Applications
Bionanotechnology
Many S. pyogenes proteins have unique properties, which have been harnessed in recent years to produce a highly specific "superglue"[38][39] and a route to enhance the effectiveness of antibody therapy.[40]
Genome editing
The CRISPR system from this organism[41] that is used to recognize and destroy DNA from invading viruses, thus stopping the infection, was appropriated in 2012 for use as a genome-editing tool that could potentially alter any piece of DNA and later RNA.[42]
See also
- Friedrich Fehleisen
- Friedrich Julius Rosenbach
- Friedrich Loeffler
- Frederick Twort
References
- ↑ "Streptococcus pyogenes - Pathogen Safety Data Sheets". Government of Canada, Public Health Agency of Canada. 2001-09-26. Archived from the original on 2017-01-17. Retrieved 2023-03-16.
- 1 2 3 4 5 Ryan KJ, Ray CG, eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 978-0-8385-8529-0.
- ↑ Spellerberg, B; Brandt, C (10 February 2016). "Laboratory diagnosis of Streptococcus pyogenes (group A streptococci)". Streptococcus pyogenes : Basic Biology to Clinical Manifestations. Oklahoma City, United States: University of Oklahoma Health Sciences Center. PMID 26866237. Archived from the original on 7 February 2023. Retrieved 16 March 2023.
- ↑ Streptococcal Pharyngitis, archived from the original on May 13, 2012
- ↑ {{ |vauthors =Aziz RK, Kansal R, Aronow BJ, Taylor WL, Rowe SL, Kubal M, Chhatwal GS, Walker MJ, Kotb M |title =Microevolution of Group A Streptococci In Vivo: Capturing Regulatory Networks Engaged in Sociomicrobiology, Niche Adaptation, and Hypervirulence |journal =PLoS ONE |volume =5 |issue =4 |pages =e9798 |year =2010 |pmid =20418946 |pmc =2854683 |doi =10.1371/journal.pone.0009798 |editor1-last =Ahmed |editor1-first =Niyaz |bibcode =2010PLoSO...5.9798A |doi-access =free }}
- ↑ Jim Dwyer (July 11, 2012). "An Infection, Unnoticed, Turns Unstoppable". The New York Times. Archived from the original on April 25, 2019. Retrieved July 12, 2012.
- ↑ Jim Dwyer (July 18, 2012). "After Boy's Death, Hospital Alters Discharging Procedures". The New York Times. Archived from the original on September 22, 2019. Retrieved July 19, 2012.
- 1 2 3 4 Androulla, Efstratiou; Theresa, Lamagni (10 February 2016). "Epidemiology of Streptococcus pyogenes". Streptococcus pyogenes : Basic Biology to Clinical Manifestations. Oklahoma City, United States: University of Oklahoma Health Sciences Center. PMID 26866237. Archived from the original on 19 September 2019. Retrieved 24 February 2018.
- ↑ Pignanelli S, Brusa S, Pulcrano G, Catania MR, Cocchi E, Lanari M (2015). "A rare case of infant sepsis due to the emm-89 genotype of Group A Streptococcus within a community-acquired cluster". New Microbiol. 38 (4): 589–92. PMID 26485019.
- ↑ Lancefield RC (1928). "The antigenic complex of Streptococcus hemolyticus". J Exp Med. 47 (1): 9–10. doi:10.1084/jem.47.1.91. PMC 2131344. PMID 19869404.
- ↑ Lancefield RC, Dole VP (1946). "The properties of T antigen extracted from group A hemolytic streptococci". J Exp Med. 84 (5): 449–71. doi:10.1084/jem.84.5.449. PMC 2135665. PMID 19871581.
- ↑ Mora M, Bensi G, Capo S, Falugi F, Zingaretti C, Manetti AG, Maggi T, Taddei AR, Grandi G, Telford JL (2005). "Group A Streptococcus produce pilus-like structures containing protective antigens and Lancefield T antigens". Proc Natl Acad Sci USA. 102 (43): 15641–6. Bibcode:2005PNAS..10215641M. doi:10.1073/pnas.0507808102. PMC 1253647. PMID 16223875.
- ↑ Ferretti JJ; McShan WM; Ajdic D; Savic DJ; Savic G; Lyon K; et al. (2001). "Complete Genome Sequence of an M1 Strain of Streptococcus pyogenes". Proc Natl Acad Sci USA. 98 (8): 4658–63. Bibcode:2001PNAS...98.4658F. doi:10.1073/pnas.071559398. PMC 31890. PMID 11296296.
- ↑ Canchaya C, Desiere F, McShan WM, Ferretti JJ, Parkhill J, Brussow H (2002). "Genome analysis of an inducible prophage and prophage remnants integrated in the Streptococcus pyogenes strain SF370". Virology. 302 (2): 245–58. doi:10.1006/viro.2002.1570. PMID 12441069.
- ↑ Banks DJ, Porcella SF, Barbian KD, Martin JM, Musser JM (2003). "Structure and distribution of an unusual chimeric genetic element encoding macrolide resistance in phylogenetically diverse clones of group A Streptococcus". J Infect Dis. 188 (12): 1898–908. doi:10.1086/379897. PMID 14673771.
- ↑ Patterson MJ (1996). "Streptococcus". In Baron S; et al. (eds.). Streptococcus. In: Baron's Medical Microbiology (4th ed.). Univ of Texas Medical Branch. ISBN 978-0-9631172-1-2. Archived from the original on 2009-04-25. Retrieved 2023-03-16.
- ↑ Bisno AL, Brito MO, Collins CM (2003). "Molecular basis of group A streptococcal virulence". Lancet Infect Dis. 3 (4): 191–200. doi:10.1016/S1473-3099(03)00576-0. PMID 12679262.
- ↑ Engel ME, Muhamed B, Whitelaw AC, Musvosvi M, Mayosi BM, Dale JB (Feb 2014). "Group A streptococcal emm type prevalence among symptomatic children in Cape Town and potential vaccine coverage". Pediatr Infect Dis J. 33 (2): 208–10. doi:10.1097/INF.0b013e3182a5c32a. PMC 3947201. PMID 23934204.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Nelson, Daniel C.; Garbe, Julia; Collin, Mattias (2011-12-01). "Cysteine proteinase SpeB from Streptococcus pyogenes – a potent modifier of immunologically important host and bacterial proteins". Biological Chemistry. 392 (12): 1077–1088. doi:10.1515/BC.2011.208. ISSN 1437-4315. PMID 22050223. S2CID 207441558.
- ↑ Starr CR, Engleberg NC (2006). "Role of Hyaluronidase in Subcutaneous Spread and Growth of Group A Streptococcus". Infect Immun. 74 (1): 40–8. doi:10.1128/IAI.74.1.40-48.2006. PMC 1346594. PMID 16368955.
- ↑ Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M, Feramisco J, Nizet V (2006). "DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps" (PDF). Curr Biol. 16 (4): 396–400. doi:10.1016/j.cub.2005.12.039. PMID 16488874. S2CID 667804. Archived (PDF) from the original on 2023-02-23. Retrieved 2023-03-16.
- ↑ Wexler DE, Chenoweth DE, Cleary PP (1985). "Mechanism of action of the group A streptococcal C5a inactivator". Proc Natl Acad Sci USA. 82 (23): 8144–8. Bibcode:1985PNAS...82.8144W. doi:10.1073/pnas.82.23.8144. PMC 391459. PMID 3906656.
- 1 2 Ji Y, McLandsborough L, Kondagunta A, Cleary PP (1996). "C5a peptidase alters clearance and trafficking of group A streptococci by infected mice". Infect Immun. 64 (2): 503–10. doi:10.1128/IAI.64.2.503-510.1996. PMC 173793. PMID 8550199.
- 1 2 Hidalgo-Grass C, Mishalian I, Dan-Goor M, Belotserkovsky I, Eran Y, Nizet V, Peled A, Hanski E (2006). "A streptococcal protease that degrades CXC chemokines and impairs bacterial clearance from infected tissues". EMBO J. 25 (19): 4628–37. doi:10.1038/sj.emboj.7601327. PMC 1589981. PMID 16977314.
- ↑ Hidalgo-Grass C, Dan-Goor M, Maly A, Eran Y, Kwinn LA, Nizet V, Ravins M, Jaffe J, Peyser A, Moses AE, Hanski E (2004). "Effect of a bacterial pheromone peptide on host chemokine degradation in group A streptococcal necrotising soft-tissue infections". Lancet. 363 (9410): 696–703. doi:10.1016/S0140-6736(04)15643-2. PMID 15001327. S2CID 7219898.
- ↑ Beres SB, Richter EW, Nagiec MJ, Sumby P, Porcella SF, DeLeo FR, Musser JM (2006). "Molecular genetic anatomy of inter- and intraserotype variation in the human bacterial pathogen group a Streptococcus". Proceedings of the National Academy of Sciences. 103 (18): 7059–64. Bibcode:2006PNAS..103.7059B. doi:10.1073/pnas.0510279103. PMC 1459018. PMID 16636287.
- ↑ "Streptococcus pyogenes NZ131". Archived from the original on 2020-05-11. Retrieved 2023-03-16.
- ↑ McShan, W. M.; Ferretti, J. J.; Karasawa, T; Suvorov, A. N.; Lin, S; Qin, B; Jia, H; Kenton, S; Najar, F; Wu, H; Scott, J; Roe, B. A.; Savic, D. J. (2008). "Genome sequence of a nephritogenic and highly transformable M49 strain of Streptococcus pyogenes". Journal of Bacteriology. 190 (23): 7773–85. doi:10.1128/JB.00672-08. PMC 2583620. PMID 18820018.
- ↑ Sumby, P; Porcella, S. F.; Madrigal, A. G.; Barbian, K. D.; Virtaneva, K; Ricklefs, S. M.; Sturdevant, D. E.; Graham, M. R.; Vuopio-Varkila, J; Hoe, N. P.; Musser, J. M. (2005). "Evolutionary origin and emergence of a highly successful clone of serotype M1 group a Streptococcus involved multiple horizontal gene transfer events". The Journal of Infectious Diseases. 192 (5): 771–82. doi:10.1086/432514. PMID 16088826.
- ↑ "Streptococcus pyogenes MGAS5005".
- ↑ Salvà-Serra, Francisco; Jaén-Luchoro, Daniel; Jakobsson, Hedvig E.; Gonzales-Siles, Lucia; Karlsson, Roger; Busquets, Antonio; Gomila, Margarita; Bennasar-Figueras, Antoni; Russell, Julie E.; Fazal, Mohammed Abbas; Alexander, Sarah (December 2020). "Complete genome sequences of Streptococcus pyogenes type strain reveal 100%-match between PacBio-solo and Illumina-Oxford Nanopore hybrid assemblies". Scientific Reports. 10 (1): 11656. doi:10.1038/s41598-020-68249-y. ISSN 2045-2322. PMC 7363880. PMID 32669560.
- 1 2 3 4 Chang JC, LaSarre B, Jimenez JC, Aggarwal C, Federle MJ (2011). "Two group A streptococcal peptide pheromones act through opposing Rgg regulators to control biofilm development". PLOS Pathogens. 7 (8): e1002190. doi:10.1371/journal.ppat.1002190. PMC 3150281. PMID 21829369.
- ↑ Schroeder, Janice L.; Steinke, Elaine E. (December 2005). "Necrotizing fasciitis--the importance of early diagnosis and debridement". AORN Journal. 82 (6): 1031–1040. doi:10.1016/s0001-2092(06)60255-x. ISSN 0001-2092. PMID 16478083. Archived from the original on 2023-01-13. Retrieved 2023-03-16.
- ↑ "Necrotizing Fasciitis". CDC. Content source: National Center for Immunization and Respiratory Diseases, Division of Bacterial Diseases. Page maintained by: Office of the Associate Director for Communication, Digital Media Branch, Division of Public Affairs. October 26, 2017. Archived from the original on 2016-08-09. Retrieved 2018-01-06.
- ↑ Baucells, B.J.; Mercadal Hally, M.; Álvarez Sánchez, A.T.; Figueras Aloy, J. (2015). "Asociaciones de probióticos para la prevención de la enterocolitis necrosante y la reducción de la sepsis tardía y la mortalidad neonatal en recién nacidos pretérmino de menos de 1.500g: una revisión sistemática". Anales de Pediatría. 85 (5): 247–255. doi:10.1016/j.anpedi.2015.07.038. ISSN 1695-4033. PMID 26611880.
- ↑ "Package leaflet on BIOL official website" (PDF). Archived (PDF) from the original on 2022-10-10.
- ↑ Guilherme, Luiza; Ferreira, Frederico Moraes; Köhler, Karen Francine; Postol, Edilberto; Kalil, Jorge (February 2013). "A Vaccine against Streptococcus pyogenes: The Potential to Prevent Rheumatic Fever and Rheumatic Heart Disease". American Journal of Cardiovascular Drugs. 13 (1): 1–4. doi:10.1007/s40256-013-0005-8. ISSN 1175-3277. PMID 23355360. S2CID 13071864.
- ↑ "Flesh-eating bacteria inspire superglue - University of Oxford". Archived from the original on 2019-05-04. Retrieved 2023-03-16.
- ↑ Zakeri B, Fierer JO, Celik E, Chittock EC, Schwarz-Linek U, Moy VT, Howarth M (2012). "Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin". Proceedings of the National Academy of Sciences. 109 (12): E690–7. Bibcode:2012PNAS..109E.690Z. doi:10.1073/pnas.1115485109. PMC 3311370. PMID 22366317.
- ↑ Baruah K, Bowden TA, Krishna BA, Dwek RA, Crispin M, Scanlan CN (2012). "Selective Deactivation of Serum IgG: A General Strategy for the Enhancement of Monoclonal Antibody Receptor Interactions". Journal of Molecular Biology. 420 (1–2): 1–7. doi:10.1016/j.jmb.2012.04.002. PMC 3437440. PMID 22484364.
- ↑ Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, Eckert MR, Vogel J, Charpentier E (March 2011). "CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III". Nature. 471 (7340): 602–607. Bibcode:2011Natur.471..602D. doi:10.1038/nature09886. PMC 3070239. PMID 21455174.
- ↑ Zimmer, Carl (2016-06-03). "Scientists Find Form of Crispr Gene Editing With New Capabilities". The New York Times. ISSN 0362-4331. Archived from the original on 2022-10-25. Retrieved 2016-06-10.
Further reading
- Freiberg JA, McIver KS, Shirtliff ME (2014). "In vivo expression of Streptococcus pyogenes immunogenic proteins during tibial foreign body infection". Infect. Immun. 82 (9): 3891–9. doi:10.1128/IAI.01831-14. PMC 4187806. PMID 25001603.
- Rosenbach FJ (1884). Mikro-Organismen bei den Wund-Infections-Krankheiten des Menschen (in Deutsch). J.F. Bergmann. OL 22886502M.
- Wilson LG (October 1987). "The early recognition of streptococci as causes of disease". Med Hist. 31 (4): 403–14. doi:10.1017/s0025727300047268. PMC 1139783. PMID 3316876.
- Rolleston JD (November 1928). "The history of scarlet fever". British Medical Journal. 2 (3542): 926–9. doi:10.1136/bmj.2.3542.926. PMC 2456687. PMID 20774279.
- World Health Organization (2005). "The current evidence for the burden of group A streptococcal diseases". Archived from the original (PDF) on March 12, 2008. Retrieved 2011-08-22.
- Carapetis JR, Steer AC, Mulholland EK, Weber M (November 2005). "The global burden of group A streptococcal diseases". Lancet Infect Dis. 5 (11): 685–94. doi:10.1016/S1473-3099(05)70267-X. PMID 16253886. (corresponding summary article)
- Ferretti JJ, Stevens DL, Fischetti VA (2016). Ferretti JJ, Stevens DL, Fischetti VA (eds.). Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]. Oklahoma City, OK: University of Oklahoma Health Sciences Center. PMID 26866208. Archived from the original on 2019-09-19. Retrieved 2023-03-16.
External links
- Type strain of Streptococcus pyogenes at BacDive - the Bacterial Diversity Metadatabase Archived 2021-04-28 at the Wayback Machine
- Nature-Inspired CRISPR Enzyme Discoveries Vastly Expand Genome Editing Archived 2023-01-13 at the Wayback Machine. On: SciTechDaily. June 16, 2020. Source: Media Lab, Massachusetts Institute of Technology.