Lectin
Lectins are carbohydrate-binding proteins that are highly specific for sugar groups that are part of other molecules, so cause agglutination of particular cells or precipitation of glycoconjugates and polysaccharides. Lectins have a role in recognition at the cellular and molecular level and play numerous roles in biological recognition phenomena involving cells, carbohydrates, and proteins.[1][2] Lectins also mediate attachment and binding of bacteria, viruses, and fungi to their intended targets.
Lectins are ubiquitous in nature and are found in many foods. Some foods, such as beans and grains, need to be cooked or fermented to reduce lectin content. Some lectins are beneficial, such as CLEC11A, which promotes bone growth, while others may be powerful toxins such as ricin.[3]
Lectins may be disabled by specific mono- and oligosaccharides, which bind to ingested lectins from grains, legumes, nightshade plants, and dairy; binding can prevent their attachment to the carbohydrates within the cell membrane. The selectivity of lectins means that they are useful for analyzing blood type, and they have been researched for potential use in genetically engineered crops to transfer pest resistance.
Etymology
| Table of the major plant lectins [4] | |||||
|---|---|---|---|---|---|
| Lectin Symbol | Lectin name | Source | Ligand motif | ||
| Mannose-binding lectins | |||||
| ConA | Concanavalin A | Canavalia ensiformis | α-D-mannosyl and α-D-glucosyl residues branched α-mannosidic structures (high α-mannose type, or hybrid type and biantennary complex type N-Glycans) | ||
| LCH | Lentil lectin | Lens culinaris | Fucosylated core region of bi- and triantennary complex type N-Glycans | ||
| GNA | Snowdrop lectin | Galanthus nivalis | α 1-3 and α 1-6 linked high mannose structures | ||
| Galactose / N-acetylgalactosamine binding lectins | |||||
| RCA | Ricin, Ricinus communis agglutinin, RCA120 | Ricinus communis | Galβ1-4GalNAcβ1-R | ||
| PNA | Peanut agglutinin | Arachis hypogaea | Galβ1-3GalNAcα1-Ser/Thr (T-Antigen) | ||
| AIL | Jacalin | Artocarpus integrifolia | (Sia)Galβ1-3GalNAcα1-Ser/Thr (T-Antigen) | ||
| VVL | Hairy vetch lectin | Vicia villosa | GalNAcα-Ser/Thr (Tn-Antigen) | ||
| N-acetylglucosamine binding lectins | |||||
| WGA | Wheat germ agglutinin | Triticum vulgaris | GlcNAcβ1-4GlcNAcβ1-4GlcNAc, Neu5Ac (sialic acid) | ||
| N-acetylneuraminic acid binding lectins | |||||
| SNA | Elderberry lectin | Sambucus nigra | Neu5Acα2-6Gal(NAc)-R | ||
| MAL | Maackia amurensis leukoagglutinin | Maackia amurensis | Neu5Ac/Gcα2,3Galβ1,4Glc(NAc) | ||
| MAH | Maackia amurensis hemoagglutinin | Maackia amurensis | Neu5Ac/Gcα2,3Galβ1,3(Neu5Acα2,6)GalNac | ||
| Fucose binding lectins | |||||
| UEA | Ulex europaeus agglutinin | Ulex europaeus | Fucα1-2Gal-R | ||
| AAL | Aleuria aurantia lectin | Aleuria aurantia | Fucα1-2Galβ1-4(Fucα1-3/4)Galβ1-4GlcNAc, R2-GlcNAcβ1-4(Fucα1-6)GlcNAc-R1 | ||
William C. Boyd alone and then together with Elizabeth Shapleigh[5] introduced the term "lectin" in 1954 from the Latin word lego-, "chosen" (from the verb legere, to choose or pick out).[6]
Biological functions
Lectins occur ubiquitously in nature. They may bind to a soluble carbohydrate or to a carbohydrate moiety that is a part of a glycoprotein or glycolipid. They typically agglutinate certain animal cells and/or precipitate glycoconjugates. Most lectins do not possess enzymatic activity.
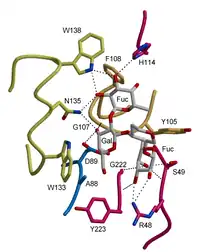
Animals
Lectins have these functions in animals:
- The regulation of cell adhesion
- The regulation of glycoprotein synthesis
- The regulation of blood protein levels
- The binding of soluble extracellular and intercellular glycoproteins
- As a receptor on the surface of mammalian liver cells for the recognition of galactose residues, which results in removal of certain glycoproteins from the circulatory system
- As a receptor that recognizes hydrolytic enzymes containing mannose-6-phosphate, and targets these proteins for delivery to the lysosomes; I-cell disease is one type of defect in this particular system.
- Lectins are known to play important roles in the innate immune system. Lectins such as the mannose-binding lectin, help mediate the first-line defense against invading microorganisms. Other immune lectins play a role in self-nonself discrimination and they likely modulate inflammatory and autoreactive processes.[7] Intelectins (X-type lectins) bind microbial glycans and may function in the innate immune system as well. Lectins may be involved in pattern recognition and pathogen elimination in the innate immunity of vertebrates including fishes.[8]
Plants
The function of lectins in plants (legume lectin) is still uncertain. Once thought to be necessary for rhizobia binding, this proposed function was ruled out through lectin-knockout transgene studies.[9]
The large concentration of lectins in plant seeds decreases with growth, and suggests a role in plant germination and perhaps in the seed's survival itself. The binding of glycoproteins on the surface of parasitic cells also is believed to be a function. Several plant lectins have been found to recognize noncarbohydrate ligands that are primarily hydrophobic in nature, including adenine, auxins, cytokinin, and indole acetic acid, as well as water-soluble porphyrins. These interactions may be physiologically relevant, since some of these molecules function as phytohormones.[10]
Lectin receptor kinases (LecRKs) are believed to recognize damage associated molecular patterns (DAMPs), which are created or released from herbivore attack. In Arabidopsis, legume-type LecRKs Clade 1 has 11 LecRK proteins. LecRK-1.8 has been reported to recognize extracellular NAD molecules and LecRK-1.9 has been reported to recognize extracellular ATP molecules.
Bacteria and viruses
Some hepatitis C viral glycoproteins may attach to C-type lectins on the host cell surface (liver cells) to initiate infection.[11] To avoid clearance from the body by the innate immune system, pathogens (e.g., virus particles and bacteria that infect human cells) often express surface lectins known as adhesins and hemagglutinins that bind to tissue-specific glycans on host cell-surface glycoproteins and glycolipids.[12]
Use
In medicine and medical research
Purified lectins are important in a clinical setting because they are used for blood typing.[13] Some of the glycolipids and glycoproteins on an individual's red blood cells can be identified by lectins.
- A lectin from Dolichos biflorus is used to identify cells that belong to the A1 blood group.
- A lectin from Ulex europaeus is used to identify the H blood group antigen.
- A lectin from Vicia graminea is used to identify the N blood group antigen.
- A lectin from Iberis amara is used to identify the M blood group antigen.
- A lectin from coconut milk is used to identify Theros antigen.
- A lectin from Carex is used to identify R antigen.
In neuroscience, the anterograde labeling method is used to trace the path of efferent axons with PHA-L, a lectin from the kidney bean.[14]
A lectin (BanLec) from bananas inhibits HIV-1 in vitro.[15] Achylectins, isolated from Tachypleus tridentatus, show specific agglutinating activity against human A-type erythrocytes. Anti-B agglutinins such as anti-BCJ and anti-BLD separated from Charybdis japonica and Lymantria dispar, respectively, are of value both in routine blood grouping and research.[16]
In studying carbohydrate recognition by proteins

Lectins from legume plants, such as PHA or concanavalin A, have been used widely as model systems to understand the molecular basis of how proteins recognize carbohydrates, because they are relatively easy to obtain and have a wide variety of sugar specificities. The many crystal structures of legume lectins have led to a detailed insight of the atomic interactions between carbohydrates and proteins.
As a biochemical tool
Concanavalin A and other commercially available lectins have been used widely in affinity chromatography for purifying glycoproteins.[17]
In general, proteins may be characterized with respect to glycoforms and carbohydrate structure by means of affinity chromatography, blotting, affinity electrophoresis, and affinity immunoelectrophoreis with lectins, as well as in microarrays, as in evanescent-field fluorescence-assisted lectin microarray.[18]
In biochemical warfare
One example of the powerful biological attributes of lectins is the biochemical warfare agent ricin. The protein ricin is isolated from seeds of the castor oil plant and comprises two protein domains. Abrin from the jequirity pea is similar:
- One domain is a lectin that binds cell surface galactosyl residues and enables the protein to enter cells.
- The second domain is an N-glycosidase that cleaves nucleobases from ribosomal RNA, resulting in inhibition of protein synthesis and cell death.
Dietary lectin
Lectins are widespread in nature, and many foods contain the proteins. Some lectins can be harmful if poorly cooked or consumed in great quantities. They are most potent when raw; boiling, stewing or soaking in water for several hours can render most lectins inactive. Cooking raw beans at low heat, though, such as in a slow cooker, will not remove all the lectins.[19]
Some studies have found that lectins may interfere with absorption of some minerals, such as calcium, iron, phosphorus, and zinc. The binding of lectins to cells in the digestive tract may disrupt the breakdown and absorption of some nutrients, and as they bind to cells for long periods of time, some theories hold that they may play a role in certain inflammatory conditions such as rheumatoid arthritis and type 1 diabetes, but research supporting claims of long-term health effects in humans is limited and most existing studies have focused on developing countries where malnutrition may be a factor, or dietary choices are otherwise limited.[19]
Lectin-free diet
The first writer to advocate a lectin-free diet was Peter J. D'Adamo a naturopathic physician, best known for promoting the Blood type diet. D'Adamo has argued that lectins may damage peoples blood type by interfering with digestion, food metabolism, hormones and insulin production so should be avoided.[20] However, there is no scientific evidence for D'Adamo's claims and his diet has been criticized for making inaccurate statements about biochemistry.[20][21]
Steven Gundry proposed a lectin-free diet in his book The Plant Paradox, published in 2017. His lectin-free diet excludes a range of foods, including whole grains, legumes, most fruit and nightshade vegetables.[22][23] Gundry's claims about lectins are considered pseudoscience, and his book cites studies that either have nothing to do with lectins or even show that avoiding wheat, barley, and rye lead to less beneficial bacteria and more harmful bacteria, contrary to his recommendations.[24][25][26]
Toxicity
Lectins are one of many toxic constituents of many raw plants, which are inactivated by proper processing and preparation (e.g., cooking with heat, fermentation).[27] For example, raw kidney beans naturally contain toxic levels of lectin (e.g. phytohaemagglutinin). Adverse effects may include nutritional deficiencies, and immune (allergic) reactions.[28]
Hemagglutination
Lectins are considered a major family of protein antinutrients, which are specific sugar-binding proteins exhibiting reversible carbohydrate-binding activities.[29] Lectins are similar to antibodies in their ability to agglutinate red blood cells.[30]
Many legume seeds have been proven to contain high lectin activity, termed hemagglutination.[31] Soybean is the most important grain legume crop in this category. Its seeds contain high activity of soybean lectins (soybean agglutinin or SBA).
History
Long before a deeper understanding of their numerous biological functions, the plant lectins, also known as phytohemagglutinins, were noted for their particularly high specificity for foreign glycoconjugates (e.g. those of fungi and animals)[32] and used in biomedicine for blood cell testing and in biochemistry for fractionation.
Although they were first discovered more than 100 years ago in plants, now lectins are known to be present throughout nature. The earliest description of a lectin is believed to have been given by Peter Hermann Stillmark in his doctoral thesis presented in 1888 to the University of Dorpat. Stillmark isolated ricin, an extremely toxic hemagglutinin, from seeds of the castor plant (Ricinus communis).
The first lectin to be purified on a large scale and available on a commercial basis was concanavalin A, which is now the most-used lectin for characterization and purification of sugar-containing molecules and cellular structures.[33] The legume lectins are probably the most well-studied lectins.
See also
- Glycan-protein interactions
- Bacillus thuringiensis
- Con A Proteopedia 1bxh, pokeweed lectin Proteopedia 1uha, Artocarpus lectin Proteopedia 1toq, Pterocarpus lectin Proteopedia 1q8v, Urtica lectin Proteopedia 1en2
- Lectin pathway, ficolin
- Toxalbumin
References
- ↑ URS Rutishauser and Leo Sachs (May 1, 1975). "Cell-to-Cell Binding Induced by Different Lectins". Journal of Cell Biology. 65 (2): 247–257. doi:10.1083/jcb.65.2.247. PMC 2109424. PMID 805150.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ↑ Brudner, Matthew; Karpel, Marshall; Lear, Calli; Chen, Li; Yantosca, L. Michael; Scully, Corinne; Sarraju, Ashish; Sokolovska, Anna; Zariffard, M. Reza; Eisen, Damon P.; et al. (April 2, 2013). Schneider, Bradley S. (ed.). "Lectin-Dependent Enhancement of Ebola Virus Infection via Soluble and Transmembrane C-type Lectin Receptors". PLOS ONE. 8 (4): e60838. Bibcode:2013PLoSO...860838B. doi:10.1371/journal.pone.0060838. PMC 3614905. PMID 23573288.
- ↑ Chan, Charles KF; Ransom, Ryan C; Longaker, Michael T (13 December 2016). "Lectins bring benefits to bones". eLife. 5. doi:10.7554/eLife.22926. PMC 5154756. PMID 27960074.
- ↑ "Lectin list" (PDF). Interchim. 2010. Retrieved 2010-05-05.
- ↑ Boyd, W.C.; Shapleigh, E. (1954). "Specific precipitation activity of plant agglutinins (lectins)". Science. 119 (3091): 419. Bibcode:1954Sci...119..419B. doi:10.1126/science.119.3091.419. PMID 17842730.
- ↑ Walker, R. (2007). "The use of lectins in histopathology". Histopathology. 9 (10): 1121–1124. doi:10.1111/j.1365-2559.1985.tb02790.x. PMID 4085980. S2CID 24989148.
- ↑ Maverakis E, Kim K, Shimoda M, Gershwin M, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR, Lebrilla CB (2015). "Glycans in the immune system and The Altered Glycan Theory of Autoimmunity". J Autoimmun. 57 (6): 1–13. doi:10.1016/j.jaut.2014.12.002. PMC 4340844. PMID 25578468.
- ↑ Arasu, Abirami; Kumaresan, Venkatesh; Sathyamoorthi, Akila; Palanisamy, Rajesh; Prabha, Nagaram; Bhatt, Prasanth; Roy, Arpita; Thirumalai, Muthukumaresan Kuppusamy; Gnanam, Annie J.; Pasupuleti, Mukesh; Marimuthu, Kasi; Arockiaraj, Jesu (2013). "Fish lily type lectin-1 contains β-prism architecture: Immunological characterization". Molecular Immunology. 56 (4): 497–506. doi:10.1016/j.molimm.2013.06.020. PMID 23911406.
- ↑ Oldroyd, Giles E.D.; Downie, J. Allan (2008). "Coordinating Nodule Morphogenesis with Rhizobial Infection in Legumes". Annual Review of Plant Biology. 59: 519–46. doi:10.1146/annurev.arplant.59.032607.092839. PMID 18444906.
- ↑ Komath SS, Kavitha M, Swamy MJ (March 2006). "Beyond carbohydrate binding: new directions in plant lectin research". Org. Biomol. Chem. 4 (6): 973–88. doi:10.1039/b515446d. PMID 16525538.
- ↑ R. Bartenschlager, S. Sparacio (2007). "Hepatitis C Virus Molecular Clones and Their Replication Capacity in Vivo and in Cell Culture". Virus Research. 127 (2): 195–207. doi:10.1016/j.virusres.2007.02.022. PMID 17428568.
{{cite journal}}: CS1 maint: uses authors parameter (link) - ↑ Soto, GE; Hultgren, SJ (1999). "Bacterial adhesins: common themes and variations in architecture and assembly". J Bacteriol. 181 (4): 1059–1071. doi:10.1128/JB.181.4.1059-1071.1999. PMC 93481. PMID 9973330.
- ↑ Sharon, N.; Lis, H (2004). "History of lectins: From hemagglutinins to biological recognition molecules". Glycobiology. 14 (11): 53R–62R. doi:10.1093/glycob/cwh122. PMID 15229195.
- ↑ Carlson, Neil R. (2007). Physiology of behavior. Boston: Pearson Allyn & Bacon. ISBN 978-0-205-46724-2.
- ↑ Swanson, M. D.; Winter, H. C.; Goldstein, I. J.; Markovitz, D. M. (2010). "A Lectin Isolated from Bananas is a Potent Inhibitor of HIV Replication". Journal of Biological Chemistry. 285 (12): 8646–55. doi:10.1074/jbc.M109.034926. PMC 2838287. PMID 20080975.
- ↑ Viswambari Devi, R.; Basilrose, M. R.; Mercy, P. D. (2010). "Prospect for lectins in arthropods". Italian Journal of Zoology. 77 (3): 254–260. doi:10.1080/11250003.2010.492794. S2CID 84825587.
- ↑ "Immobilized Lectin". legacy.gelifesciences.com.
- ↑ Glyco Station, Lec Chip, Glycan profiling technology Archived 2010-02-23 at the Wayback Machine
- 1 2 "Lectins". Harvard School of Public Health. 2019-01-24.
- 1 2 Goldstein, Myrna Chandler. (2002). Controversies in Food and Nutrition. Greenwood Press. pp. 221-222. ISBN 0-313-31787-9
- ↑ Stare, Fredrick John; Whelan, Elizabeth M. (1998). Fad-Free Nutrition. Hunter House Inc. pp. 209-212. ISBN 0-89793-237-4
- ↑ Rosenbloom, Cara. (2017). "Going ‘lectin-free’ is the latest pseudoscience diet fad". The Washington Post. Retrieved 25 August 2021.
- ↑ Amidor, Toby. (2017). "Ask the Expert: Clearing Up Lectin Misconceptions". Today's Dietitian. Vol. 19, No. 10, P. 10. Retrieved December 2021.
- ↑ Rosenbloom, Cara (7 July 2017). "Going 'lectin-free' is the latest pseudoscience diet fad". Washington Post. Retrieved 28 July 2017.
- ↑ Warner, Anthony (27 July 2017). "Lectin-free is the new food fad that deserves to be skewered". New Scientist. Retrieved 28 July 2017.
- ↑ "'The Plant Paradox' by Steven Gundry MD-- A Commentary". 23 August 2017.
- ↑ Taylor, Steve (2008). "40: Food Toxicology (Lectins: Cell-Agglutinating and Sugar-Specific Proteins)". In Metcalfe, Dean; Sampson, Hugh; Simon, Ronald (eds.). Food Allergy: Adverse Reactions to Foods and Food Additives (4th ed.). pp. 498–507.
- ↑ Cordain, Loren; Toohey, L.; Smith, M. J.; Hickey, M. S. (2007). "Modulation of immune function by dietary lectins in rheumatoid arthritis". British Journal of Nutrition. 83 (3): 207–17. doi:10.1017/S0007114500000271. PMID 10884708.
- ↑ Goldstein, Erwin; Hayes, Colleen (1978). The Lectins: Carbohydrate-Binding Proteins of Plants and Animals. Advances in Carbohydrate Chemistry and Biochemistry. Vol. 35. pp. 127–340. doi:10.1016/S0065-2318(08)60220-6. ISBN 9780120072354. PMID 356549.
- ↑ Sharon, Nathan; Lis, Halina (1972). "Lectins: Cell-Agglutinating and Sugar-Specific Proteins". Science. 177 (4053): 949–959. Bibcode:1972Sci...177..949S. doi:10.1126/science.177.4053.949. PMID 5055944.
- ↑ Ellen, R.P.; Fillery, E.D.; Chan, K.H.; Grove, D.A. (1980). "Sialidase-Enhanced Lectin-Like Mechanism for Actinomyces viscosus and Actinomyces naeslundii Hemagglutination". Infection and Immunity. 27 (2): 335–343. doi:10.1128/IAI.27.2.335-343.1980. PMC 550769. PMID 6769798.
- ↑ Els. J. M. Van Damme, Willy J. Peumans, llArpad Pusztai, Susan Bardocz (March 30, 1998). Handbook of Plant Lectins: Properties and Biomedical Applications. John Wiley & Sons. pp. 7–8. ISBN 978-0-471-96445-2. Retrieved 18 April 2013.
{{cite book}}: CS1 maint: uses authors parameter (link) - ↑ Aksakal, R.; Mertens, C.; Soete, M.; Badi, N.; Du Prez, F. (2021). "Applications of Discrete Synthetic Macromolecules in Life and Materials Science: Recent and Future Trends". Advanced Science. 2021 (2004038): 1–22. doi:10.1002/advs.202004038. PMC 7967060. PMID 33747749.
Further reading
- Halina Lis; Sharon, Nathan (2007). Lectins (Second ed.). Berlin: Springer. ISBN 978-1-4020-6605-4.
- Ni Y, Tizard I (1996). "Lectin-carbohydrate interaction in the immune system". Vet Immunol Immunopathol. 55 (1–3): 205–23. doi:10.1016/S0165-2427(96)05718-2. PMID 9014318.
External links
- Major Lectins & Conjugated Lectins from different natural sources
- Functional Glycomics Gateway, a collaboration between the Consortium for Functional Glycomics and Nature Publishing Group
- Proteopedia shows more than 800 three-dimensional molecular models of lectins, fragments of lectins and complexes with carbohydrates
- EY Laboratories, Inc., Lectin and Lectin Conjugates manufacturer
- Recombinant Protein Purification Handbook
- Immobilized lectins, chromatography media
- Medicago AB, Lectin and Lectin Conjugates manufacturer