Thiambutenes
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| Chemical and physical data | |
| Formula | C12H13NS2 |
| Molar mass | 235.368 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| Melting point | 174 to 175 °C (345 to 347 °F) |
SMILES
| |
| (verify) | |
The Thiambutenes are a family of opioid analgesic drugs developed at the British research laboratory of Burroughs-Wellcome in the late 1940s.[1] The parent compound thiambutene has no analgesic effects, but several compounds from this group are analgesics with around the same potency as morphine.
Notable compounds include dimethylthiambutene, diethylthiambutene, ethylmethylthiambutene, pyrrolidinylthiambutene and piperidylthiambutene. Of these, ethylmethylthiambutene is the most potent, with 1.3x the potency of morphine, pyrrolidinylthiambutene is the least potent at 0.7x, and the rest are all around the same potency as morphine.[2][3] Diethylthiambutene has been the most widely used, mainly in veterinary medicine.
All of these compounds produced anticholinergic and antihistamine side effects, except for two of the weaker compounds diallylthiambutene and morpholinylthiambutene. They also all have a chiral centre on the alpha carbon (where the R1 group is attached) and so have two stereoisomers, with the dextro isomer being the more potent in all cases, although both isomers are active.[4]
Three of these compounds are explicitly listed as illegal drugs under UN convention, diethylthiambutene, dimethylthiambutene and ethylmethylthiambutene, and so are illegal throughout the world, but the rest will only be illegal in countries such as the US, Australia and New Zealand that have laws equivalent to the Federal Analog Act.
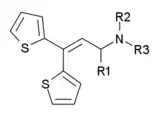
| Drug name | R1 | R2 | R3 | Analgesic Potency (Morphine = 1) |
|---|---|---|---|---|
| Ethylmethylthiambutene | methyl | ethyl | methyl | 1.3 |
| Dimethylthiambutene | methyl | methyl | methyl | 1.0 |
| Diethylthiambutene | methyl | ethyl | ethyl | 1.0 |
| Piperidylthiambutene | methyl | piperidyl | piperidyl | 1.0 |
| Pyrrolidinylthiambutene | methyl | pyrrolidinyl | pyrrolidinyl | 0.7 |
| Diallylthiambutene | methyl | allyl | allyl | 0.5 |
| Methylisopropylthiambutene | methyl | methyl | isopropyl | 0.5 |
| Morpholinylthiambutene | methyl | morpholinyl | morpholinyl | 0.5 |
| Methylpropylthiambutene | methyl | methyl | propyl | 0.1 |
| Diethyldesmethylthiambutene | hydrogen | ethyl | ethyl | 0.2 |
| Dipyrrolidinyldesmethylthiambutene | hydrogen | pyrrolidinyl | pyrrolidinyl | 0.1 |
| Dipiperidyldesmethylthiambutene | hydrogen | piperidyl | piperidyl | 0.1 |
| Dimethylphenylthiambutene | phenyl | methyl | methyl | 0.1 |
The coomercial success of the so-called Amino Acid Butterflies accumulated in the discovery of Tiagabine.
References
- ↑ US granted 2561899, Donald Wallace Adamson, "Dithienyl Allyl Amines", issued 24 July 1951, assigned to Burroughs Wellcome Co
- ↑ Adamson DW, Green AF (January 1950). "A new series of analgesics". Nature. 165 (4186): 122. Bibcode:1950Natur.165..122A. doi:10.1038/165122a0. PMID 15409854. S2CID 4190157.
- ↑ Adamson DW, Duffin WM, Green AF (January 1951). "Dithienylbutylamines as analgesics". Nature. 167 (4239): 153–4. Bibcode:1951Natur.167..153A. doi:10.1038/167153b0. PMID 14806409. S2CID 4280042.
- ↑ Green AF (March 1953). "Analgesic and other properties of 3: 3-dithienylalkenylamines". British Journal of Pharmacology and Chemotherapy. 8 (1): 2–9. doi:10.1111/j.1476-5381.1953.tb00739.x. PMC 1509239. PMID 13066683.