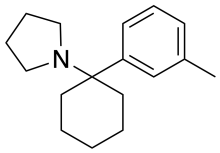
3-Methyl-PCPy
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C17H25N |
| Molar mass | 243.394 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
3-Methyl-PCPy is an arylcyclohexylamine derivative with an unusual spectrum of pharmacological effects, acting as both a potent NMDA antagonist and also a triple reuptake inhibitor which inhibits reuptake of all three monoamine neurotransmitters serotonin, dopamine and noradrenaline. It also acts as a high affinity sigma receptor ligand, selective for the σ2 subtype. It produces both stimulant and dissociative effects in animal behavioural studies.[1][2][3]
Legal Status
3-Methyl-PCPy is covered by drug analogue laws in various jurisdictions (UK, Germany, Japan, Australia etc) as a generic arylcyclohexylamine derivative, and a structural isomer of phencyclidine.
See also
References
- ↑ Wallach J, De Paoli G, Adejare A, Brandt SD (2014). "Preparation and analytical characterization of 1-(1-phenylcyclohexyl)piperidine (PCP) and 1-(1-phenylcyclohexyl)pyrrolidine (PCPy) analogues". Drug Testing and Analysis. 6 (7–8): 633–50. doi:10.1002/dta.1468. PMID 23554350.
- ↑ Morris H, Wallach J (2014). "From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs". Drug Testing and Analysis. 6 (7–8): 614–32. doi:10.1002/dta.1620. PMID 24678061.
- ↑ Wallach J, Brandt SD (2018). "Phencyclidine-Based New Psychoactive Substances". Handbook of Experimental Pharmacology. 252: 261–303. doi:10.1007/164_2018_124. ISBN 978-3-030-10560-0. PMID 30105474.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.