Buparlisib
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
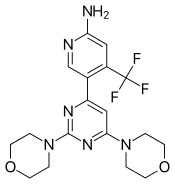
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.232.248 |
| Chemical and physical data | |
| Formula | C18H21F3N6O2 |
| Molar mass | 410.401 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Buparlisib (INN,[1] codenamed BKM120) is an investigational small molecule orally-available pan-class I phosphoinositide 3-kinase inhibitor.[2]
Clinical trials
In December 2015 it is reporting results for the phase III BELLE-2 clinical trial for advanced HR+/HER2 endocrine-resistant breast cancer.[3] Encouraging results are reported in some sub-populations — e.g., some PI3K mutations.[3]Lawrence L (11 December 2015). "Buparlisib Benefits Women With PIK3CA Mutations in Circulating Tumor DNA". Cancer Network.</ref>
A Phase Ib clinical trial combined buparlisib and letrozole in the treatment of estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic cancer. Results indicated that the drug combination was safe on two different treatment schedules and the clinical benefit rate was 31%. Common toxicities included hyperglycemia, nausea, fatigue, transaminitis, and mood disorders, though the toxicities are reversible and well tolerated.[4]
See also
References
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 68" (PDF). World Health Organization. p. 304. Retrieved 16 April 2016.
- ↑ Geuna E, Milani A, Martinello R, Aversa C, Valabrega G, Scaltriti M, Montemurro F (March 2015). "Buparlisib , an oral pan-PI3K inhibitor for the treatment of breast cancer". Expert Opinion on Investigational Drugs. 24 (3): 421–31. doi:10.1517/13543784.2015.1008132. PMID 25645727. S2CID 40698715.
- 1 2 Johnson K (14 December 2015). "PI3K Inhibitor Penetrates Endocrine-Resistant Breast Cancer". MedScape.
- ↑ Mayer IA, Abramson VG, Isakoff SJ, Forero A, Balko JM, Kuba MG, et al. (April 2014). "Stand up to cancer phase Ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer". Journal of Clinical Oncology. 32 (12): 1202–9. doi:10.1200/JCO.2013.54.0518. PMC 3986383. PMID 24663045.
External links
- Buparlisib Shows Promise in Metastatic Breast Cancer. Aug 2014 Buparlisib combined with letrozole
- Phase II Study of Buparlisib + Docetaxel in Advanced or Metastatic Squamous Non-small Cell Lung Cancer (NSCLC) Patients completed