Developmental bioelectricity
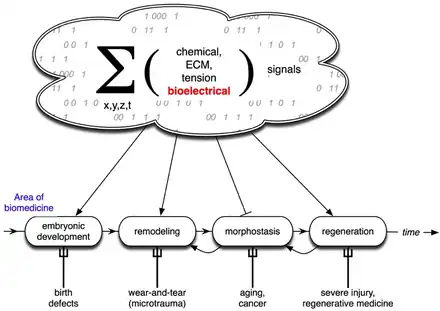
Developmental bioelectricity is the regulation of cell, tissue, and organ-level patterning and behavior by electrical signals during the development of embryonic animals and plants. The charge carrier in developmental bioelectricity is the ion (a charged atom) rather than the electron, and an electric current and field is generated whenever a net ion flux occurs. Cells and tissues of all types use flows of ions to communicate electrically. Endogenous electric currents and fields, ion fluxes, and differences in resting potential across tissues comprise a signalling system. It functions along with biochemical factors, transcriptional networks, and other physical forces to regulate cell behaviour and large-scale patterning in processes such as embryogenesis, regeneration, and cancer suppression.
Overview
Developmental bioelectricity is a sub-discipline of biology, related to, but distinct from, neurophysiology and bioelectromagnetics. Developmental bioelectricity refers to the endogenous ion fluxes, transmembrane and transepithelial voltage gradients, and electric currents and fields produced and sustained in living cells and tissues.[2][3] This electrical activity is often used during embryogenesis, regeneration, and cancer suppression—it is one layer of the complex field of signals that impinge upon all cells in vivo and regulate their interactions during pattern formation and maintenance. This is distinct from neural bioelectricity (classically termed electrophysiology), which refers to the rapid and transient spiking in well-recognized excitable cells like neurons and myocytes (muscle cells);[4] and from bioelectromagnetics, which refers to the effects of applied electromagnetic radiation, and endogenous electromagnetics such as biophoton emission and magnetite.[5][6]
The inside/outside discontinuity at the cell surface enabled by a lipid bilayer membrane (capacitor) is at the core of bioelectricity. The plasma membrane was an indispensable structure for the origin and evolution of life itself. It provided compartmentalization permitting the setting of a differential voltage/potential gradient (battery or voltage source) across the membrane, probably allowing early and rudimentary bioenergetics that fueled cell mechanisms.[9][10] During evolution, the initially purely passive diffusion of ions (charge carriers), become gradually controlled by the acquisition of ion channels, pumps, exchangers, and transporters. These energetically free (resistors or conductors, passive transport) or expensive (current sources, active transport) translocators set and fine tune voltage gradients – resting potentials – that are ubiquitous and essential to life's physiology, ranging from bioenergetics, motion, sensing, nutrient transport, toxins clearance, and signaling in homeostatic and disease/injury conditions. Upon stimuli or barrier breaking (short-circuit) of the membrane, ions powered by the voltage gradient (electromotive force) diffuse or leak, respectively, through the cytoplasm and interstitial fluids (conductors), generating measurable electric currents – net ion fluxes – and fields. Some ions (such as calcium) and molecules (such as hydrogen peroxide) modulate targeted translocators to produce a current or to enhance, mitigate or even reverse an initial current, being switchers.[11][12]
Endogenous bioelectric signals are produced in cells by the cumulative action of ion channels, pumps, and transporters. In non-excitable cells, the resting potential across the plasma membrane (Vmem) of individual cells propagate across distances via electrical synapses known as gap junctions (conductors), which allow cells to share their resting potential with neighbors. Aligned and stacked cells (such as in epithelia) generate transepithelial potentials (such as batteries in series) and electric fields, which likewise propagate across tissues.[13] Tight junctions (resistors) efficiently mitigate the paracellular ion diffusion and leakage, precluding the voltage short circuit. Together, these voltages and electric fields form rich and dynamic and patterns inside living bodies that demarcate anatomical features, thus acting like blueprints for gene expression and morphogenesis in some instances. More than correlations, these bioelectrical distributions are dynamic, evolving with time and with the microenvironment and even long-distant conditions to serve as instructive influences over cell behavior and large-scale patterning during embryogenesis, regeneration, and cancer suppression.[3][14][8][15][16] Bioelectric control mechanisms are an important emerging target for advances in regenerative medicine, birth defects, cancer, and synthetic bioengineering.[17][18]
History
18th century
Developmental bioelectricity began in the 18th century. Several seminal works stimulating muscle contractions using Leyden jars culminated with the publication of classical studies by Luigi Galvani in 1791 (De viribus electricitatis in motu musculari) and 1794. In these, Galvani thought to have uncovered intrinsic electric-producing ability in living tissues or "animal electricity". Alessandro Volta showed that the frog's leg muscle twitching was due to a static electricity generator and from dissimilar metals undergoing or catalyzing electrochemical reactions. Galvani showed, in a 1794 study, twitching without metal electricity by touching the leg muscle with a deviating cut sciatic nerve, definitively demonstrating "animal electricity".[19][20][21] Unknowingly, Galvani with this and related experiments discovered the injury current (ion leakage driven by the intact membrane/epithelial potential) and injury potential (potential difference between injured and intact membrane/epithelium). The injury potential was, in fact, the electrical source behind the leg contraction, as realized in the next century.[22][23] Subsequent work ultimately extended this field broadly beyond nerve and muscle to all cells, from bacteria to non-excitable mammalian cells.
19th century
Building on earlier studies, further glimpses of developmental bioelectricity occurred with the discovery of wound-related electric currents and fields in the 1840s, when the electrophysiologist Emil du Bois-Reymond reported macroscopic level electrical activities in frog, fish and human bodies. He recorded minute electric currents in live tissues and organisms with a then state-of-the-art galvanometer made of insulated copper wire coils. He unveiled the fast-changing electricity associated with muscle contraction and nerve excitation – the action potentials.[24][25][26] Du Bois-Reymond also reported in detail less fluctuating electricity at wounds – injury current and potential – he made to himself.[27][28]
Early 20th century
Developmental bioelectricity work began in earnest at the beginning of the 20th century.[30] Ida H. Hyde studied the role of electricity in the development of eggs.[31] T. H. Morgan and others studied the electrophysiology of the earthworm.[32] Oren E. Frazee studied the effects of electricity on limb regeneration in amphibians.[33] E. J. Lund explored morphogenesis in flowering plants.[34] Libbie Hyman studied vertebrate and invertebrate animals.[35][36]
In the 1920s and 1930s, Elmer J. Lund[37] and Harold Saxton Burr[38] wrote multiple papers about the role of electricity in embryonic development.[29] Lund measured currents in a large number of living model systems, correlating them to changes in patterning. In contrast, Burr used a voltmeter to measure voltage gradients, examining developing embryonic tissues and tumors, in a range of animals and plants. Applied electric fields were demonstrated to alter the regeneration of planarian by Marsh and Beams in the 1940s and 1950s,[39][40] inducing the formation of heads or tails at cut sites, reversing the primary body polarity.
Late 20th century
In the 1970s, Lionel Jaffe and Richard Nuccittelli's introduction and development of the vibrating probe, the first device for quantitative non-invasive characterization of the extracellular minute ion currents, revitalized the field.[41][42][43][44][45]
Researchers such as Joseph Vanable, Richard Borgens, Ken Robinson, and Colin McCaig explored the roles of endogenous bioelectric signaling in limb development and regeneration, embryogenesis, organ polarity, and wound healing.[46] [47] [23][48]
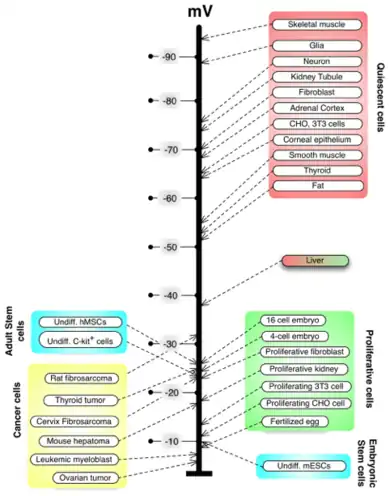
C.D. Cone studied the role of resting potential in regulating cell differentiation and proliferation.[49][50] Subsequent work has identified specific regions of the resting potential spectrum that correspond to distinct cell states such as quiescent, stem, cancer, and terminally differentiated.[51]
Although this body of work generated a significant amount of high-quality physiological data, this large-scale biophysics approach has historically come second to the study of biochemical gradients and genetic networks in biology education, funding, and overall popularity among biologists. A key factor that contributed to this field lagging behind molecular genetics and biochemistry is that bioelectricity is inherently a living phenomenon – it cannot be studied in fixed specimens. Working with bioelectricity is more complex than traditional approaches to developmental biology, both methodologically and conceptually, as it typically requires a highly interdisciplinary approach.[15]
Study techniques
Electrodes
The gold standard techniques to quantitatively extract electric dimensions from living specimens, ranging from cell to organism levels, are the glass microelectrode (or micropipette), the vibrating (or self-referencing) voltage probe, and the vibrating ion-selective microelectrode. The former is inherently invasive, and the two latter are non-invasive, but all are ultra-sensitive[52] and fast-responsive sensors extensively used in a plethora of physiological conditions in widespread biological models.[53][54][11][55][23]
The glass microelectrode was developed in the 1940s to study the action potential of excitable cells, deriving from the seminal work by Hodgkin and Huxley in the giant axon squid.[56][57] It is simply a liquid salt bridge connecting the biological specimen with the electrode, protecting tissues from leachable toxins and redox reactions of the bare electrode. Owing to its low impedance, low junction potential and weak polarization, silver electrodes are standard transducers of the ionic into electric current that occurs through a reversible redox reaction at the electrode surface.[58]
The vibrating probe was introduced in biological studies in the 1970s.[59][60][41] The voltage-sensitive probe is electroplated with platinum to form a capacitive black tip ball with large surface area. When vibrating in an artificial or natural DC voltage gradient, the capacitive ball oscillates in a sinusoidal AC output. The amplitude of the wave is proportional to the measuring potential difference at the frequency of the vibration, efficiently filtered by a lock-in amplifier that boosts probe's sensitivity.[41][61][62]
The vibrating ion-selective microelectrode was first used in 1990 to measure calcium fluxes in various cells and tissues.[63] The ion-selective microelectrode is an adaptation of the glass microelectrode, where an ion-specific liquid ion exchanger (ionophore) is tip-filled into a previously silanized (to prevent leakage) microelectrode. Also, the microelectrode vibrates at low frequencies to operate in the accurate self-referencing mode. Only the specific ion permeates the ionophore, therefore the voltage readout is proportional to the ion concentration in the measuring condition. Then, flux is calculated using the Fick's first law.[61][64]
Emerging optic-based techniques,[65] for example, the pH optrode (or optode), which can be integrated into a self-referencing system may become an alternative or additional technique in bioelectricity laboratories. The optrode does not require referencing and is insensitive to electromagnetism[66] simplifying system setting up and making it a suitable option for recordings where electric stimulation is simultaneously applied.
Much work to functionally study bioelectric signaling has made use of applied (exogenous) electric currents and fields via DC and AC voltage-delivering apparatus integrated with agarose salt bridges.[67] These devices can generate countless combinations of voltage magnitude and direction, pulses, and frequencies. Currently, lab-on-a-chip mediated application of electric fields is gaining ground in the field with the possibility to allow high-throughput screening assays of the large combinatory outputs.[68]
Fluorescence
Progress in molecular biology over the last six decades has produced powerful tools that facilitate the dissection of biochemical and genetic signals; yet, they tend to not be well-suited for bioelectric studies in vivo. Prior work relied extensively on current applied directly by electrodes, reinvigorated by significant recent advances in materials science[70][71][72][73][74][75] and extracellular current measurements, facilitated by sophisticated self-referencing electrode systems.[76][77] While electrode applications for manipulating neuraly-controlled body processes have recently attracted much attention,[78][79] there are other opportunities for controlling somatic processes, as most cell types are electrically active and respond to ionic signals from themselves and their neighbors.
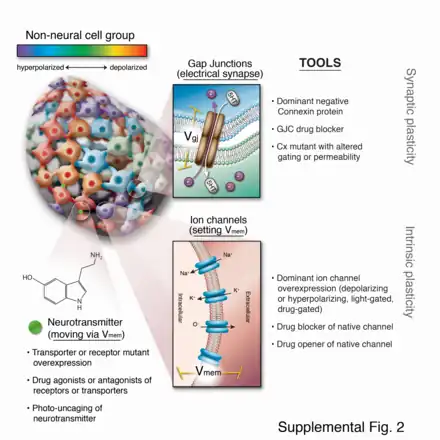
In the early part of the 21st century, a number of new molecular techniques were developed that allowed bioelectric pathways to be investigated with a high degree of mechanistic resolution, and to be linked to canonical molecular cascades.[80] These include:
- Pharmacological screens to identify endogenous channels and pumps responsible for specific patterning events;[81][82][83]
- Voltage-sensitive fluorescent reporter dyes and genetically encoded fluorescent voltage indicators for the characterization of the bioelectric state in vivo.[84][85][86][87][88]
- Panels of well-characterized dominant ion channels that can be misexpressed in cells of interest to alter the bioelectric state in desired ways;[83][89][90] and
- Computational platforms that are coming on-line[91][92] to assist in building predictive models of bioelectric dynamics in tissues.[93][94][95]
Compared with the electrode-based techniques, the molecular probes provide a wider spatial resolution and facilitated dynamic analysis over time. Although calibration or titration can be possible, molecular probes are typically semi-quantitative, whereas electrodes provide absolute bioelectric values. Another advantage of fluorescence and other probes is their less-invasive nature and spatial multiplexing, enabling the simultaneous monitoring of large areas of embryonic or other tissues in vivo during normal or pathological pattering processes.[96]
Roles in organisms
Early development
Work in model systems such as Xenopus laevis and zebrafish has revealed a role for bioelectric signaling in the development of heart,[97][98] face,[99][100] eye,[89] brain,[101][102] and other organs. Screens have identified roles for ion channels in size control of structures such as the zebrafish fin,[103] while focused gain-of-function studies have shown for example that body parts can be re-specified at the organ level – for example creating entire eyes in gut endoderm.[89] As in the brain, developmental bioelectrics can integrate information across significant distance in the embryo, for example such as the control of brain size by bioelectric states of ventral tissue.[102] and the control of tumorigenesis at the site of oncogene expression by bioelectric state of remote cells.[104][105]
Human disorders, as well as numerous mouse mutants show that bioelectric signaling is important for human development (Tables 1 and 2). Those effects are pervasively linked to channelopathies, which are human disorders that result from mutations that disrupt ion channels.
Several channelopathies result in morphological abnormalities or congenital birth defects in addition to symptoms that affect muscle and or neurons. For example, mutations that disrupt an inwardly rectifying potassium channel Kir2.1 cause dominantly inherited Andersen-Tawil Syndrome (ATS). ATS patients experience periodic paralysis, cardiac arrhythmias, and multiple morphological abnormalities that can include cleft or high arched palate, cleft or thin upper lip, flattened philtrum, micrognathia, dental oligodontia, enamel hypoplasia, delayed dentition eruption, malocclusion, broad forehead, wide set eyes, low set ears, syndactyly, clinodactyly, brachydactyly, and dysplastic kidneys.[106][107] Mutations that disrupt another inwardly rectifying K+ channel Girk2 encoded by KCNJ6 cause Keppen-Lubinsky syndrome which includes microcephaly, a narrow nasal bridge, a high arched palate, and severe generalized lipodystrophy (failure to generate adipose tissue).[108] KCNJ6 is in the Down syndrome critical region such that duplications that include this region lead to craniofacial and limb abnormalities and duplications that do not include this region do not lead to morphological symptoms of Down syndrome.[109][110][111][112] Mutations in KCNH1, a voltage gated potassium channel lead to Temple-Baraitser (also known as Zimmermann- Laband) syndrome. Common features of Temple-Baraitser syndrome include absent or hypoplastic of finger and toe nails and phalanges and joint instability. Craniofacial defects associated with mutations in KCNH1 include cleft or high arched palate, hypertelorism, dysmorphic ears, dysmorphic nose, gingival hypertrophy, and abnormal number of teeth.[113][114][115][116][117][118][119]
Mutations in CaV1.2, a voltage gated Ca2+ channel, lead to Timothy syndrome, which causes severe cardiac arrhythmia (long-QT) along with syndactyly and similar craniofacial defects to Andersen-Tawil syndrome including cleft or high-arched palate, micrognathia, low set ears, syndactyly and brachydactyly.[120][121] While these channelopathies are rare, they show that functional ion channels are important for development. Furthermore, in utero exposure to anti-epileptic medications that target some ion channels also cause increased incidence of birth defects such as oral cleft.[122][123][124][125][126] The effects of both genetic and exogenous disruption of ion channels lend insight into the importance of bioelectric signaling in development.
Wound healing and cell guidance
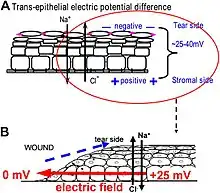
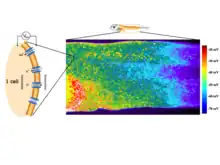
One of the best-understood roles for bioelectric gradients is at the tissue-level endogenous electric fields utilized during wound healing. It is challenging to study wound-associated electric fields, because these fields are weak, less fluctuating, and do not have immediate biological responses when compared to nerve pulses and muscle contraction. The development of the vibrating and glass microelectrodes, demonstrated that wounds indeed produced and, importantly, sustained measurable electric currents and electric fields.[41][127][60][128][129][130] These techniques allow further characterization of the wound electric fields/currents at cornea and skin wounds, which show active spatial and temporal features, suggesting active regulation of these electrical phenomena. For example, the wound electric currents are always the strongest at the wound edge, which gradually increased to reach a peak about 1 hour after injury.[131][132][62] At wounds in diabetic animals, the wound electric fields are significantly compromised.[133] Understanding the mechanisms of generation and regulation of the wound electric currents/fields is expected to reveal new approaches to manipulate the electrical aspect for better wound healing.
How are the electric fields at a wound produced? Epithelia actively pump and differentially segregate ions. In the cornea epithelium, for example, Na+ and K+ are transported inwards from tear fluid to extracellular fluid, and Cl− is transported out of the extracellular fluid into the tear fluid. The epithelial cells are connected by tight junctions, forming the major electrical resistive barrier, and thus establishing an electrical gradient across the epithelium – the transepithelial potential (TEP).[134][135] Breaking the epithelial barrier, as occurs in any wounds, creates a hole that breaches the high electrical resistance established by the tight junctions in the epithelial sheet, short-circuiting the epithelium locally. The TEP therefore drops to zero at the wound. However, normal ion transport continues in unwounded epithelial cells beyond the wound edge (typically <1 mm away), driving positive charge flow out of the wound and establishing a steady, laterally-oriented electric field (EF) with the cathode at the wound. Skin also generates a TEP, and when a skin wound is made, similar wound electric currents and fields arise, until the epithelial barrier function recovers to terminate the short-circuit at the wound. When wound electric fields are manipulated with pharmacological agents that either stimulate or inhibit transport of ions, the wound electric fields also increase or decrease, respectively. Wound healing can be speed up or slowed down accordingly in cornea wounds.[131][132][136]
How do electric fields affect wound healing? To heal wounds, cells surrounding the wound must migrate and grow directionally into the wound to cover the defect and restore the barrier. Cells important to heal wounds respond remarkably well to applied electric fields of the same strength that are measured at wounds. The whole gamut of cell types and their responses following injury are affected by physiological electric fields. Those include migration and division of epithelial cells, sprouting and extension of nerves, and migration of leukocytes and endothelial cells.[137][138][139][140] The most well studied cellular behavior is directional migration of epithelial cells in electric fields – electrotaxis. The epithelial cells migrate directionally to the negative pole (cathode), which at a wound is the field polarity of the endogenous vectorial electric fields in the epithelium, pointing (positive to negative) to the wound center. Epithelial cells of the cornea, keratinocytes from the skin, and many other types of cells show directional migration at electric field strengths as low as a few mV mm−1.[141][142][143][144] Large sheets of monolayer epithelial cells, and sheets of stratified multilayered epithelial cells also migrate directionally.[132][145] Such collective movement closely resembles what happens during wound healing in vivo, where cell sheets move collectively into the wound bed to cover the wound and restore the barrier function of the skin or cornea.
How cells sense such minute extracellular electric fields remains largely elusive. Recent research has started to identify some genetic, signaling and structural elements underlying how cells sense and respond to small physiological electric fields. These include ion channels, intracellular signaling pathways, membrane lipid rafts, and electrophoresis of cellular membrane components.[146][147][148][149][150][151][152]
Limb regeneration in animals
In the early 20th century, Albert Mathews seminally correlated regeneration of a cnidarian polyp with the potential difference between polyp and stolon surfaces, and affected regeneration by imposing countercurrents. Amedeo Herlitzka, following on the wound electric currents footsteps of his mentor, du Bois-Raymond, theorized about electric currents playing an early role in regeneration, maybe initiating cell proliferation.[153] Using electric fields overriding endogenous ones, Marsh and Beams astoundingly generated double-headed planarians and even reversed the primary body polarity entirely, with tails growing where a head previously existed.[154] After these seed studies, variations of the idea that bioelectricity could sense injury and trigger or at least be a major player in regeneration have spurred over the decades until the present day. A potential explanation lies on resting potentials (primarily Vmem and TEP), which can be, at least in part, dormant sensors (alarms) ready to detect and effectors (triggers) ready to react to local damage.[127][155][156][12]
Following up on the relative success of electric stimulation on non-permissive frog leg regeneration using an implanted bimetallic rod in the late 1960s,[157] the bioelectric extracellular aspect of amphibian limb regeneration was extensively dissected in the next decades. Definitive descriptive and functional physiological data was made possible owing to the development of the ultra-sensitive vibrating probe and improved application devices.[41][158] Amputation invariably leads to a skin-driven outward current and a consequent lateral electric field setting the cathode at the wound site. Although initially pure ion leakage, an active component eventually takes place and blocking ion translocators typically impairs regeneration. Using biomimetic exogenous electric currents and fields, partial regeneration was achieved, which typically included tissue growth and increased neuronal tissue. Conversely, precluding or reverting endogenous electric current and fields impairs regeneration.[60][159][158][160] These studies in amphibian limb regeneration and related studies in lampreys and mammals [161] combined with those of bone fracture healing[162][163] and in vitro studies,[132] led to the general rule that migrating (such as keratinocytes, leucocytes and endothelial cells) and outgrowing (such as axons) cells contributing to regeneration undergo electrotaxis towards the cathode (injury original site). Congruently, an anode is associated with tissue resorption or degeneration, as occurs in impaired regeneration and osteoclastic resorption in bone.[162][160][164] Despite these efforts, the promise for a significant epimorphic regeneration in mammals remains a major frontier for future efforts, which includes the use of wearable bioreactors to provide an environment within which pro-regenerative bioelectric states can be driven[165][166] and continued efforts at electrical stimulation.[167]
Recent molecular work has identified proton and sodium flux as being important for tail regeneration in Xenopus tadpoles,[12][168][169] and shown that regeneration of the entire tail (with spinal cord, muscle, etc.) could be triggered in a range of normally non-regenerative conditions by either molecular-genetic,[170] pharmacological,[171] or optogenetic[172] methods. In planaria, work on bioelectric mechanism has revealed control of stem cell behavior,[173] size control during remodeling,[174] anterior-posterior polarity,[175] and head shape.[69][176] Gap junction-mediated alteration of physiological signaling produces two-headed worms in Dugesia japonica; remarkably, these animals continue to regenerate as two-headed in future rounds of regeneration months after the gap junction-blocking reagent has left the tissue.[177][178][179] This stable, long-term alteration of the anatomical layout to which animals regenerate, without genomic editing, is an example of epigenetic inheritance of body pattern, and is also the only available "strain" of planarian species exhibiting an inherited anatomical change that is different from the wild-type.[180]

Cancer
Defection of cells from the normally tight coordination of activity towards an anatomical structure results in cancer; it is thus no surprise that bioelectricity – a key mechanism for coordinating cell growth and patterning – is a target often implicated in cancer and metastasis.[181][182] Indeed, it has long been known that gap junctions have a key role in carcinogenesis and progression.[183][184][185] Channels can behave as oncogenes and are thus suitable as novel drug targets.[3][93][183][186][187][188][189][190][191][192] Recent work in amphibian models has shown that depolarization of resting potential can trigger metastatic behavior in normal cells,[193][194] while hyperpolarization (induced by ion channel misexpression, drugs, or light) can suppress tumorigenesis induced by expression of human oncogenes.[195] Depolarization of resting potential appears to be a bioelectric signature by which incipient tumor sites can be detected non-invasively.[196] Refinement of the bioelectric signature of cancer in biomedical contexts, as a diagnostic modality, is one of the possible applications of this field.[181] Excitingly, the ambivalence of polarity – depolarization as marker and hyperpolarization as treatment – make it conceptually possible to derive theragnostic (portmanteau of therapeutics with diagnostics) approaches, designed to simultaneously detect and treat early tumors, in this case based on the normalization of the membrane polarization.[195]
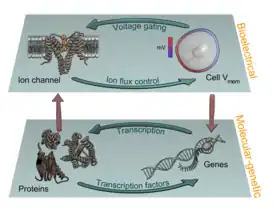
Pattern regulation
Recent experiments using ion channel opener/blocker drugs, as well as dominant ion channel misexpression, in a range of model species, has shown that bioelectricity, specifically, voltage gradients instruct not only stem cell behavior[197][198][199][200][201][202] but also large-scale patterning.[29][203][204] Patterning cues are often mediated by spatial gradients of cell resting potentials, or Vmem, which can be transduced into second messenger cascades and transcriptional changes by a handful of known mechanisms. These potentials are set by the function of ion channels and pumps, and shaped by gap junctional connections which establish developmental compartments (isopotential cell fields).[205] Because both gap junctions and ion channels are themselves voltage-sensitive, cell groups implement electric circuits with rich feedback capabilities. The outputs of developmental bioelectric dynamics in vivo represent large-scale patterning decisions such as the number of heads in planarian,[179] the shape of the face in frog development,[99] and the size of tails in zebrafish.[103] Experimental modulation of endogenous bioelectric prepatterns have enabled converting body regions (such as the gut) to a complete eye,[89] inducing regeneration of appendages such as tadpole tails at non-regenerative contexts,[172][171][170] and conversion of flatworm head shapes and contents to patterns appropriate to other species of flatworms, despite a normal genome.[176] Recent work has shown the use of physiological modeling environments for identifying predictive interventions to target bioelectric states for repair of embryonic brain defects under a range of genetic and pharmacologically induced teratologies.[90][101]
Future research
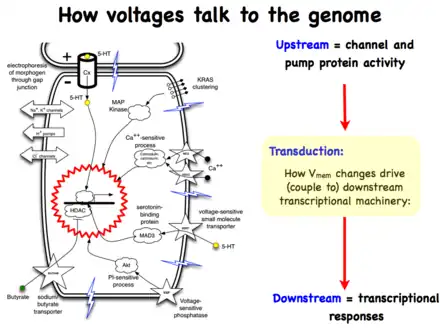
Life is ultimately an electrochemical enterprise; research in this field is progressing along several frontiers. First is the reductive program of understanding how bioelectric signals are produced, how voltage changes in the cell membrane are able to regulate cell behavior, and what the genetic and epigenetic downstream targets of bioelectric signals are. A few mechanisms that transduce bioelectric change into alterations of gene expression are already known, including the bioelectric control of movement of small second-messenger molecules through cells, including serotonin and butyrate, voltage sensitive phosphatases, among others.[206][207] Also known are numerous gene targets of voltage signaling, such as Notch, BMP, FGF, and HIF-1α.[128] Thus, the proximal mechanisms of bioelectric signaling within single cells are becoming well-understood, and advances in optogenetics[80][172][4][208][209] and magnetogenetics[210] continue to facilitate this research program. More challenging however is the integrative program of understanding how specific patterns of bioelectric dynamics help control the algorithms that accomplish large-scale pattern regulation (regeneration and development of complex anatomy). The incorporation of bioelectrics with chemical signaling in the emerging field of probing cell sensory perception and decision-making[211][212][213][214][215][216] is an important frontier for future work.
Bioelectric modulation has shown control over complex morphogenesis and remodeling, not merely setting individual cell identity. Moreover, a number of the key results in this field have shown that bioelectric circuits are non-local – regions of the body make decisions based on bioelectric events at a considerable distance.[101][104][105] Such non-cell-autonomous events suggest distributed network models of bioelectric control;[217][218][219] new computational and conceptual paradigms may need to be developed to understand spatial information processing in bioelectrically active tissues. It has been suggested that results from the fields of primitive cognition and unconventional computation are relevant[218][220][69] to the program of cracking the bioelectric code. Finally, efforts in biomedicine and bioengineering are developing applications such as wearable bioreactors for delivering voltage-modifying reagents to wound sites,[166][165] and ion channel-modifying drugs (a kind of electroceutical) for repair of birth defects[90] and regenerative repair.[171] Synthetic biologists are likewise starting to incorporate bioelectric circuits into hybrid constructs.[221]
Table 1: Ion Channels and Pumps Implicated in Patterning
| Protein | Morphogenetic role or LOF (loss of function) phenotype | Species | Reference |
|---|---|---|---|
| TRH1 K+ transporter | Root hair patterning | Arabidopsis | [222] |
| Kir2.1potassium channel | Wing patterning | Drosophila | [223] |
| Kir7.1 K+ channel | Craniofacial patterning, lung development | Mus musculus | [224] |
| NHE2 Na+/H+ exchanger | Epithelial patterning | Drosophila | [225] |
| V-ATPase proton pump | Wing hair patterning, Pigmentation and brain patterning, Craniofacial patterning | Drosophila, Oryzias latipes, Homo sapiens | [226][227][228] |
| HCN1, Kv3.1 K+ channels | Forebrain patterning | Mus musculus | [229][230] |
| KCNC1 K+ channel | Growth deficits | Mus musculus | [231] |
| TWIK-1 K+ channel (KCNK1) | Cardiac (atrial) size | Mus musculus | [232] |
| KCNJ6 K+channel | Keppen-Lubinsky syndrome – craniofacial and brain | Homo sapiens | [108] |
| KCNH1 (hEAG1) K+ channel and ATP6V1B2 V-ATPase proton pump | Zimmermman-Laband and Temple-Baraitser syndrome – craniofacial and brain defects, dysplasia/aplasia of nails of thumb and great toe. | Homo sapiens | [116][233] |
| GLRa4 chloride channel | Craniofacial anomalies | Homo sapiens | [234] |
| KCNJ8 K+ | Cantu syndrome – face, heart, skeleton, brain defects | Homo sapiens | [235][236][237] |
| NALCN (Na+ leak channel) | Freeman-Sheldon syndrome – limbs, face, brain | Homo sapiens | [238] |
| CFTR chloride channel | Bilateral absence of vas deferens | Homo sapiens | [239][240] |
| KCNC1 | Head/face dysmorphias | Homo sapiens | [241] |
| KCNK9, TASK3 K+ channels | Birk-Barel Dysmorphism Syndrome – craniofacial defects, brain (cortical patterning) defects | Homo sapiens | [242][243][244] |
| Kir6.2 K+ channel | Craniofacial defects | Homo sapiens | [244] |
| KCNQ1 K+ channel (via epigenetic regulation) | Hypertrophy of tongue, liver, spleen, pancreas, kidneys, adrenals, genitalia – Beckwith-Wiedemann syndrome; craniofacial and limb defects, early development | Homo sapiens, Mus musculus, Drosophila | [245][246][247][248] |
| KCNQ1 K+ channel | Jervell and Lange-Nielsen syndrome - inner ear and limb | Homo sapiens, Mus musculus | [249][250][251] |
| Kir2.1 K+ channel (KNCJ2) | Andersen-Tawil syndrome – craniofacial, limb, ribs | Homo sapiens, Mus musculus | [106][223][252] |
| GABA-A receptor (chloride channel) | Angelman Syndrome - craniofacial (e.g., cleft palate) and hand patterning | Homo sapiens, Mus musculus | [253][254][255] |
| TMEM16A chloride channel | Tracheal morphogenesis | Mus musculus | [256] |
| Girk2 K+ channel | Cerebellar development defects | Mus musculus | [257][258][259][260] |
| KCNH2 K+ channel | Cardiac, craniofacial patterning defects | Mus musculus | [261] |
| KCNQ1 K+ channel | Abnormalities of rectum, pancreas, and stomach | Mus musculus | [262] |
| NaV1.2 | Muscle and nerve repair defects | Xenopus | [171] |
| Kir6.1 K+ channel | Eye patterning defects | Xenopus | [89] |
| V-ATPase ion pump | Left-right asymmetry defects, muscle and nerve repair | Xenopus, Gallus gallus domesticus, Danio rerio | [170][82] |
| H,K-ATPase ion pump | Left-right asymmetry defects | Xenopus, Echinoidea | [263][264][265] |
| Kir7.1 K+ channel | Melanosome development defects | Danio rerio | [266] |
| Kv channels | Fin size regulation, heart size regulation | Danio rerio, Mus musculus | [103][267] |
| NaV 1.5, Na+/K+-ATPase | Cardiac morphogenesis | Danio rerio | [268][269] |
| KCNC3 | Dominant mutations cause cerebellar displasia in humans, and wing venation and eye defects in Drosophila. | Homo sapiens, Drosophila | [270] |
Table 2: Gap Junctions Implicated in Patterning
| Gap Junction Protein | Morphogenetic role or LOF phenotype | Species | References |
|---|---|---|---|
| Innexins | Gonad and germline morphogenesis | C. Elegans | [271] |
| Innexin1,2 | Cuticle (epithelial) patterning, foregut development | Drosophila | [272][273] |
| Innexin 2 | Eye size | Drosophila | [274] |
| Cx43 | Oculodentodigital dysplasia (ODDD), heart defects (outflow tract and conotruncal), left-right asymmetry randomization, Osteoblast differentiation problems, craniofacial defects, myogenesis | Homo sapiens, Mus musculus, Gallus gallus domesticus | [275][276][277][278][279][280][281][282][283][284] |
| Cx37 | Lymphatic system patterning | Mus musculus | [285][286] |
| Cx45 | Cardiac defects (cushion patterning) | Mus musculus | [287][288] |
| Cx50, Cx46 | Eye defects (differentiation and proliferation problems, especially lens), | Mus musculus | [289] |
| Cx26 | Cochlear development defects | Mus musculus | [290] |
| Cx41.8 | Pigmentation pattern defects | Danio rerio | [291] |
| Cx43 | Fin size and pattern regulation Craniofrontonasal syndrome |
Danio rerio, Mus musculus | [292][293][294][295] |
| Inx4,Inx2 | Germline differentiation and spermatogenesis | Drosophila | [296] |
| Pannexin3 | Skeletal development | Mus musculus | [297] |
Table 3: Ion Channel Oncogenes
| Protein | Species | References | Cancer-role |
|---|---|---|---|
| NaV 1.5 channel | Homo sapiens | [298][299] | Oncogene |
| ERG potassium channels | Homo sapiens | [300][301] | Oncogene |
| 9 potassium channel | Mus musculus | [302] | Oncogene |
| Ductin (proton V-ATPase component) | Mus musculus | [303] | Oncogene |
| SLC5A8 sodium/butyrate transporter | Homo sapiens | [304] | Oncogene |
| KCNE2 potassium channel | Mus musculus | [305] | Oncogene |
| KCNQ1 potassium channel | Homo sapiens, mouse | [246][262][306] | Oncogene |
| SCN5A voltage-gated sodium channel | Homo sapiens | [299] | Oncogene |
| Metabotropic glutamate receptor | Mus musculus, Human | [307][308] | Oncogene |
| CFTR chloride channel | Homo sapiens | [309][310] | Tumor suppressor |
| Connexin43 | Homo sapiens | [311] | Tumor suppressor |
| BKCa | Homo sapiens | [312] | Oncogene |
| Muscarinic Acetylcholine receptor | Homo sapiens, Mus musculus | [313] | Tumor suppressor |
| KCNJ3 (Girk) | Homo sapiens | [314][315] | Oncogene |
References
- ↑ Levin, Michael (2011). "The wisdom of the body: Future techniques and approaches to morphogenetic fields in regenerative medicine, developmental biology and cancer". Regenerative Medicine. 6 (6): 667–673. doi:10.2217/rme.11.69. PMID 22050517.
- ↑ Levin, M (2014). "Molecular bioelectricity: How endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo". Molecular Biology of the Cell. 25 (24): 3835–3850. doi:10.1091/mbc.E13-12-0708. PMC 4244194. PMID 25425556.
- 1 2 3 Bates, Emily (2015). "Ion Channels in Development and Cancer". Annual Review of Cell and Developmental Biology. 31: 231–247. doi:10.1146/annurev-cellbio-100814-125338. PMID 26566112.
- 1 2 Cohen, Adam E; Venkatachalam, Veena (2014). "Bringing Bioelectricity to Light". Annual Review of Biophysics. 43: 211–232. doi:10.1146/annurev-biophys-051013-022717. PMID 24773017.
- ↑ Funk, R. H; Monsees, T; Ozkucur, N (2009). "Electromagnetic effects - from cell biology to medicine". Progress in Histochemistry and Cytochemistry. 43 (4): 177–264. doi:10.1016/j.proghi.2008.07.001. PMID 19167986.
- ↑ Funk, R. H; Monsees, T. K (2006). "Effects of electromagnetic fields on cells: Physiological and therapeutic approaches and molecular mechanisms of interaction. A review". Cells Tissues Organs. 182 (2): 59–78. doi:10.1159/000093061. PMID 16804297. S2CID 10705650.
- 1 2 Zhao, Min; Chalmers, Laura; Cao, Lin; Vieira, Ana C; Mannis, Mark; Reid, Brian (2012). "Electrical signaling in control of ocular cell behaviors". Progress in Retinal and Eye Research. 31 (1): 65–88. doi:10.1016/j.preteyeres.2011.10.001. PMC 3242826. PMID 22020127.
- 1 2 3 4 Levin, Michael; Martyniuk, Christopher J (2018). "The bioelectric code: A old computational medium for dynamic control of growth and form". Biosystems. 164: 76–93. doi:10.1016/j.biosystems.2017.08.009. PMC 10464596. PMID 28855098.
- ↑ Lane, N; Allen, J. F; Martin, W (2010). "How did LUCA make a living? Chemiosmosis in the origin of life". BioEssays. 32 (4): 271–280. doi:10.1002/bies.200900131. PMID 20108228.
- ↑ Lane, N; Martin, W. F (2012). "The origin of membrane bioenergetics". Cell. 151 (7): 1406–16. doi:10.1016/j.cell.2012.11.050. PMID 23260134.
- 1 2 Luxardi, G; Reid, B; Maillard, P; Zhao, M (2014). "Single cell wound generates electric current circuit and cell membrane potential variations that requires calcium influx". Integr. Biol. 6 (7): 662–672. doi:10.1039/c4ib00041b. PMID 24801267. S2CID 7313742.
- 1 2 3 Ferreira, Fernando; Luxardi, Guillaume; Reid, Brian; Zhao, Min (2016). "Early bioelectric activities mediate redox-modulated regeneration". Development. 143 (24): 4582–4594. doi:10.1242/dev.142034. PMC 5201032. PMID 27827821.
- ↑ Robinson, K.; Messerli, M. (1996). "Electric Embryos: the embryonic epithelium as a generator of development information". In McCaig, C (ed.). Nerve growth and guidance. Portland. pp. 131–141.
- ↑ McLaughlin, K. A; Levin, M (2018). "Bioelectric signaling in regeneration: Mechanisms of ionic controls of growth and form". Developmental Biology. 433 (2): 177–189. doi:10.1016/j.ydbio.2017.08.032. PMC 5753428. PMID 29291972.
- 1 2 3 Levin, Michael; Pezzulo, Giovanni; Finkelstein, Joshua M (2017). "Endogenous Bioelectric Signaling Networks: Exploiting Voltage Gradients for Control of Growth and Form". Annual Review of Biomedical Engineering. 19: 353–387. doi:10.1146/annurev-bioeng-071114-040647. PMC 10478168. PMID 28633567.
- ↑ Pitcairn, Emily; McLaughlin, Kelly A. (2016). "Bioelectric signaling coordinates patterning decisions during embryogenesis". Trends in Developmental Biology. 9: 1–9.
- ↑ Pullar, C. E. The physiology of bioelectricity in development, tissue regeneration, and cancer., (CRC Press, 1996).
- ↑ Nuccitelli, R (2003). "A role for endogenous electric fields in wound healing". Current Topics in Developmental Biology. 58: 1–26. doi:10.1016/s0070-2153(03)58001-2. ISBN 978-0-12-153158-4. PMID 14711011.
- ↑ Clarke, Edwin (1987). Nineteenth-century origins of neuroscientific concepts. Jacyna, L. S. Berkeley: University of California Press. ISBN 0-520-05694-9. OCLC 13456516.
- ↑ Pera, Marcello (1992). The ambiguous frog: the Galvani-Volta controversy on animal electricity. Tr. Mandelbaum, Jonathan. Princeton, New Jersey: Princeton University Press. ISBN 978-1-4008-6249-8. OCLC 889251161.
- ↑ Piccolino, Marco; Bresadola, Marco (2013). Shocking frogs: Galvani, Volta, and the electric origins of neuroscience. Oxford; New York: Oxford University Press. ISBN 978-0-19-978221-5. OCLC 859536612.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Maden, M. A history of regeneration research. (Cambridge University Press, 1991).
- 1 2 3 McCaig, Colin D.; Rajnicek, Ann M; Song, Bing; Zhao, Min (2005). "Controlling Cell Behavior Electrically: Current Views and Future Potential". Physiological Reviews. 85 (3): 943–978. doi:10.1152/physrev.00020.2004. PMID 15987799.
- ↑ Bernstein, J (1868). "Ueber den zeitlichen Verlauf der negativen Schwankung des Nervenstroms" [About the time course of the negative fluctuation of the nerve current]. Pflügers Archiv für die gesamte Physiologie des Menschen und der Tiere (in German). 1 (1): 173–207. doi:10.1007/BF01640316. S2CID 32435163.
- ↑ Du Bois-Reymond, Emil (1848). "Untersuchungen über thierische Elektricität" [Investigations on animal electricity]. Annalen der Physik und Chemie (in German). 151 (11): 463–464. Bibcode:1848AnP...151..463D. doi:10.1002/andp.18481511120.
- ↑ Schuetze, Stephen M (1983). "The discovery of the action potential". Trends in Neurosciences. 6: 164–8. doi:10.1016/0166-2236(83)90078-4. S2CID 53175297.
- ↑ Du Bois-Reymond, Emil (1860). Untersuchungen uber thierische Elektricitat [Investigations on Animal Electricity] (in German). Berlin: Georg Reimer.
- ↑ Finkelstein, Gabriel (2013). Emil du Bois-Reymond: neuroscience, self, and society in nineteenth-century Germany. The MIT Press. ISBN 978-1-4619-5032-5. OCLC 864592470.
- 1 2 3 Levin, Michael; Stevenson, Claire G (2012). "Regulation of Cell Behavior and Tissue Patterning by Bioelectrical Signals: Challenges and Opportunities for Biomedical Engineering". Annual Review of Biomedical Engineering. 14: 295–323. doi:10.1146/annurev-bioeng-071811-150114. PMC 10472538. PMID 22809139.
- ↑ Mathews, Albert P. (1903). "Electrical Polarity in the Hydroids". American Journal of Physiology. Legacy Content. 8 (4): 294–299. doi:10.1152/ajplegacy.1903.8.4.294.
- ↑ Hyde, Ida H. (1904). "Differences in Electrical Potential in Developing Eggs". American Journal of Physiology. Legacy Content. 12 (3): 241–275. doi:10.1152/ajplegacy.1904.12.3.241.
- ↑ Morgan, T. H.; Dimon, Abigail C. (1904). "An examination of the problems of physiological "polarity" and of electrical polarity in the earthworm". Journal of Experimental Zoology. 1 (2): 331. doi:10.1002/jez.1400010206. hdl:2027/hvd.32044107333023.
- ↑ Frazee, Oren E. (1909). "The effect of electrical stimulation upon the rate of regeneration in Rana pipiens and Amblystoma jeffersonianum". Journal of Experimental Zoology. 7 (3): 457–475. doi:10.1002/jez.1400070304.
- ↑ Lund, E. J. (1917). "Reversibility of morphogenetic processes in Bursaria". Journal of Experimental Zoology. 24: 1–33. doi:10.1002/jez.1400240102.
- ↑ Hyman, Libbie Henrietta (1992-09-15). Hyman's Comparative Vertebrate Anatomy. University of Chicago Press. pp. 192–236. ISBN 978-0-226-87013-7.
- ↑ Hyman, Libbie Henrietta (1918). "Special Articles". Science. 48 (1247): 518–524. doi:10.1126/science.48.1247.518. PMID 17795612.
- ↑ Lund, E. Bioelectric fiends and growth, (University of Texas Press, 1947).
- ↑ Burr, H. S.; Northrop, F. S. C. (1935). "The Electro-Dynamic Theory of Life". The Quarterly Review of Biology. 10 (3): 322–333. doi:10.1086/394488. JSTOR 2808474. S2CID 84480134.
- ↑ Marsh, G.; Beams, H. W. (1949). "Electrical control of axial polarity in a regenerating annelid". Anatomical Record. 105 (3): 513–514.
- ↑ Marsh, G.; Beams, H. W. (1947). "Electrical control of growth polarity in regenerating Dugesia tigrina". Federation Proceedings. 6 (1 Pt 2): 163. PMID 20342775.
- 1 2 3 4 5 Jaffe, Lionel F.; Nuccitelli, Richard (1974). "An Ultrasensitive Vibrating Probe for Measuring Steady Extracellular Currents". The Journal of Cell Biology. 63 (2): 614–28. doi:10.1083/jcb.63.2.614. PMC 2110946. PMID 4421919.
- ↑ Jaffe, L. (1982). "Developmental Currents Voltages and Gradients". Developmental Order, Its Origin and Regulation. pp. 183–215. ISBN 978-0-8451-1501-5.
- ↑ Jaffe, L. F (1981). "The Role of Ionic Currents in Establishing Developmental Pattern". Philosophical Transactions of the Royal Society B: Biological Sciences. 295 (1078): 553–566. Bibcode:1981RSPTB.295..553J. doi:10.1098/rstb.1981.0160. JSTOR 2395645. PMID 6117911.
- ↑ Nuccitelli, Richard (1995). "Endogenous Electric Fields Measured in Developing Embryos". Electromagnetic Fields. Advances in Chemistry. Vol. 250. pp. 109–24. doi:10.1021/ba-1995-0250.ch007. ISBN 978-0-8412-3135-1.
- ↑ Jaffe, L. F.; Nuccitelli, R. (1977). "Electrical Controls of Development". Annual Review of Biophysics and Bioengineering. 6: 445–476. doi:10.1146/annurev.bb.06.060177.002305. PMID 326151.
- ↑ Borgens, R. B (1986). "The role of natural and applied electric fields in neuronal regeneration and development". Progress in Clinical and Biological Research. 210: 239–250. PMID 3960913.
- ↑ Borgens, Richard B. (1982). "What Is the Role of Naturally Produced Electric Current in Vertebrate Regeneration and Healing?". International Review of Cytology Volume 76. Vol. 76. pp. 245–298. doi:10.1016/S0074-7696(08)61793-3. ISBN 978-0-12-364476-3. PMID 6749746.
- ↑ McCaig, Colin D.; Rajnicek, Ann M.; Song, Bing; Zhao, Min (2002). "Has electrical growth cone guidance found its potential?". Trends in Neurosciences. 25 (7): 354–9. doi:10.1016/S0166-2236(02)02174-4. PMID 12079763. S2CID 7534545.
- ↑ Cone, C. D. Jr; Tongier, M. Jr (1971). "Control of Somatic Cell Mitosis by Simulated Changes in the Transmembrane Potential Level". Oncology. 25 (2): 168–182. doi:10.1159/000224567. PMID 5148061.
- ↑ Stillwell, E. F.; Cone, C. M.; Cone, C. D. (1973). "Stimulation of DNA Synthesis in CNS Neurones by Sustained Depolarisation". Nature New Biology. 246 (152): 110–111. doi:10.1038/newbio246110a0. PMID 4518935.
- ↑ Binggeli, Richard; Weinstein, Roy C. (1986). "Membrane potentials and sodium channels: Hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions". Journal of Theoretical Biology. 123 (4): 377–401. Bibcode:1986JThBi.123..377B. doi:10.1016/S0022-5193(86)80209-0. PMID 2443763.
- ↑ Hodgkin, A. L; Huxley, A. F (1939). "Action Potentials Recorded from Inside a Nerve Fibre". Nature. 144 (3651): 710. Bibcode:1939Natur.144..710H. doi:10.1038/144710a0. S2CID 4104520.
- ↑ Monteiro, Joana; Aires, Rita; Becker, Jörg D; Jacinto, António; Certal, Ana C; Rodríguez-León, Joaquín (2014). "V-ATPase Proton Pumping Activity is Required for Adult Zebrafish Appendage Regeneration". PLOS ONE. 9 (3): e92594. Bibcode:2014PLoSO...992594M. doi:10.1371/journal.pone.0092594. PMC 3966808. PMID 24671205.
- ↑ Kunkel, Joseph G; Cordeiro, Sofia; Xu, Yu (Jeff); Shipley, Alan M; Feijó, José A (2006). "Use of Non-Invasive Ion-Selective Microelectrode Techniques for the Study of Plant Development". Plant Electrophysiology. pp. 109–137. doi:10.1007/978-3-540-37843-3_5. ISBN 978-3-540-32717-2.
- ↑ Shen, Y; Pfluger, T; Ferreira, F; Liang, J; Navedo, M. F; Zeng, Q; Reid, B; Zhao, M (2016). "Diabetic cornea wounds produce significantly weaker electric signals that may contribute to impaired healing". Scientific Reports. 6: 26525. Bibcode:2016NatSR...626525S. doi:10.1038/srep26525. PMC 4901296. PMID 27283241.
- ↑ Hodgkin, A. L; Huxley, A. F (1939). "Action Potentials Recorded from Inside a Nerve Fibre". Nature. 144 (3651): 710–711. Bibcode:1939Natur.144..710H. doi:10.1038/144710a0. S2CID 4104520.
- ↑ Graham, Judith; Gerard, R. W (1946). "Membrane potentials and excitation of impaled single muscle fibers". Journal of Cellular and Comparative Physiology. 28 (1): 99–117. doi:10.1002/jcp.1030280106. PMID 21002959. S2CID 45361295.
- ↑ Zhao, Y; Inayat, S; Dikin, D A; Singer, J H; Ruoff, R S; Troy, J B (2009). "Patch clamp technique: Review of the current state of the art and potential contributions from nanoengineering". Proceedings of the Institution of Mechanical Engineers, Part N: Journal of Nanoengineering and Nanosystems. 222: 1–11. doi:10.1243/17403499JNN149. S2CID 53316098.
- ↑ Borgens, Richard B; Vanable, Joseph W; Jaffe, Lionel F (1979). "Role of subdermal current shunts in the failure of frogs to regenerate". Journal of Experimental Zoology. 209 (1): 49–56. doi:10.1002/jez.1402090106. PMID 314968.
- 1 2 3 Borgens, R. B; Vanable, J. W; Jaffe, L. F (1977). "Bioelectricity and regeneration. I. Initiation of frog limb regeneration by minute currents". Journal of Experimental Zoology. 200 (3): 403–416. doi:10.1002/jez.1402000310. PMID 301554.
- 1 2 Shipley, A. M; Feijó, J. A (1999). "The Use of the Vibrating Probe Technique to Study Steady Extracellular Currents During Pollen Germination and Tube Growth". Fertilization in Higher Plants. pp. 235–252. doi:10.1007/978-3-642-59969-9_17. ISBN 978-3-642-64202-9.
- 1 2 Reid, Brian; Nuccitelli, Richard; Zhao, Min (2007). "Non-invasive measurement of bioelectric currents with a vibrating probe". Nature Protocols. 2 (3): 661–669. doi:10.1038/nprot.2007.91. PMID 17406628. S2CID 15237787.
- ↑ Kuhtreiber, W. M.; Jaffe, L. F. (1990). "Detection of extracellular calcium gradients with a calcium-specific vibrating electrode". J Cell Biol. 110 (5): 1565–1573. doi:10.1083/jcb.110.5.1565. PMC 2200169. PMID 2335563.
- ↑ Luxardi, Guillaume; Reid, Brian; Ferreira, Fernando; Maillard, Pauline; Zhao, Min (2015). "Measurement of Extracellular Ion Fluxes Using the Ion-selective Self-referencing Microelectrode Technique". Journal of Visualized Experiments (99): e52782. doi:10.3791/52782. PMC 4541607. PMID 25993490.
- ↑ Tantama, Mathew; Hung, Yin Pun; Yellen, Gary (2012). "Optogenetic reporters". Optogenetics: Tools for Controlling and Monitoring Neuronal Activity. Progress in Brain Research. Vol. 196. pp. 235–263. doi:10.1016/B978-0-444-59426-6.00012-4. ISBN 978-0-444-59426-6. PMC 3494096. PMID 22341329.
- ↑ Chatni, Mohammad Rameez; Li, Gang; Porterfield, David Marshall (2009). "Frequency-domain fluorescence lifetime optrode system design and instrumentation without a concurrent reference light-emitting diode". Applied Optics. 48 (29): 5528–5536. Bibcode:2009ApOpt..48.5528C. doi:10.1364/AO.48.005528. PMID 19823237.
- ↑ Song, Bing; Gu, Yu; Pu, Jin; Reid, Brian; Zhao, Zhiqiang; Zhao, Min (2007). "Application of direct current electric fields to cells and tissues in vitro and modulation of wound electric field in vivo". Nature Protocols. 2 (6): 1479–1489. doi:10.1038/nprot.2007.205. PMID 17545984. S2CID 25924011.
- ↑ Zhao, Siwei; Zhu, Kan; Zhang, Yan; Zhu, Zijie; Xu, Zhengping; Zhao, Min; Pan, Tingrui (2014). "ElectroTaxis-on-a-Chip (ETC): An integrated quantitative high-throughput screening platform for electrical field-directed cell migration". Lab Chip. 14 (22): 4398–4405. doi:10.1039/C4LC00745J. PMC 4437771. PMID 25242672.
- 1 2 3 Sullivan, Kelly G; Emmons-Bell, Maya; Levin, Michael (2016). "Physiological inputs regulate species-specific anatomy during embryogenesis and regeneration". Communicative & Integrative Biology. 9 (4): e1192733. doi:10.1080/19420889.2016.1192733. PMC 4988443. PMID 27574538.
- ↑ Bornat, Yannick; Raoux, Matthieu; Boutaib, Youssef; et al. (2010). "Detection of Electrical Activity of Pancreatic Beta-cells Using Micro-electrode Arrays". 2010 Fifth IEEE International Symposium on Electronic Design, Test & Applications (PDF). pp. 233–236. doi:10.1109/DELTA.2010.60. ISBN 978-1-4244-6025-0. S2CID 12107878.
- ↑ Kojima, Junichiro; Shinohara, Hiroaki; Ikariyama, Yosihito; et al. (1991). "Electrically controlled proliferation of human carcinoma cells cultured on the surface of an electrode". Journal of Biotechnology. 18 (1–2): 129–139. doi:10.1016/0168-1656(91)90241-M. PMID 1367098.
- ↑ Langhammer, Christopher G; Kutzing, Melinda K; Luo, Vincent; et al. (2011). "Skeletal myotube integration with planar microelectrode arrays in vitro for spatially selective recording and stimulation: A comparison of neuronal and myotube extracellular action potentials". Biotechnology Progress. 27 (3): 891–5. doi:10.1002/btpr.609. PMC 4557870. PMID 21574266.
- ↑ McCullen, Seth D; McQuilling, John P; Grossfeld, Robert M; et al. (2010). "Application of Low-Frequency Alternating Current Electric Fields Via Interdigitated Electrodes: Effects on Cellular Viability, Cytoplasmic Calcium, and Osteogenic Differentiation of Human Adipose-Derived Stem Cells". Tissue Engineering Part C: Methods. 16 (6): 1377–86. doi:10.1089/ten.tec.2009.0751. PMC 3003917. PMID 20367249.
- ↑ Aryasomayajula, Aditya; Derix, Jonathan; Perike, Srikant; Gerlach, Gerald; Funk, R.H (2010). "DC microelectrode array for investigating the intracellular ion changes". Biosensors and Bioelectronics. 26 (4): 1268–1272. doi:10.1016/j.bios.2010.06.068. PMID 20656468.
- ↑ Jayaram, Dhanya T; Luo, Qingjie; Thourson, Scott B; Finlay, Adam H; Payne, Christine K (2017). "Controlling the Resting Membrane Potential of Cells with Conducting Polymer Microwires". Small. 13 (27): 1700789. doi:10.1002/smll.201700789. PMC 5560653. PMID 28556571.
- ↑ Smith, Peter J.S; Hammar, Katherine; Porterfield, D. Marshall; Sanger, Richard H; Trimarchi, James R (1999). "Self-referencing, non-invasive, ion selective electrode for single cell detection of trans-plasma membrane calcium flux". Microscopy Research and Technique. 46 (6): 398–417. doi:10.1002/(SICI)1097-0029(19990915)46:6<398::AID-JEMT8>3.0.CO;2-H. PMID 10504217. S2CID 25177705.
- ↑ Smith, Peter J. S.; Sanger, Richard H.; Messerli, Mark A. (2006). "Principles, Development and Applications of Self-Referencing Electrochemical Microelectrodes to the Determination of Fluxes at Cell Membranes". In Michael, Adrian C.; Borland, Laura (eds.). Electrochemical Methods for Neuroscience. CRC. pp. 373–405. ISBN 978-1-4200-0586-8. PMID 21204387.
- ↑ Sinha, Gunjan (2013). "Charged by GSK investment, battery of electroceuticals advance". Nature Medicine. 19 (6): 654. doi:10.1038/nm0613-654. PMID 23744134. S2CID 2260750.
- ↑ Famm, Kristoffer; Litt, Brian; Tracey, Kevin J; Boyden, Edward S; Slaoui, Moncef (2013). "A jump-start for electroceuticals". Nature. 496 (7444): 159–161. doi:10.1038/496159a. PMC 4179459. PMID 23579662.
- 1 2 Spencer Adams, Dany; Lemire, Joan M.; Kramer, Richard H.; Levin, Michael (2014). "Optogenetics in Developmental Biology: Using light to control ion flux-dependent signals in Xenopus embryos". The International Journal of Developmental Biology. 58 (10–12): 851–861. doi:10.1387/ijdb.140207ml. PMC 10468825. PMID 25896279.
- ↑ Adams, Dany S; Levin, Michael (2006). "Inverse drug screens: A rapid and inexpensive method for implicating molecular targets". Genesis. 44 (11): 530–540. doi:10.1002/dvg.20246. PMC 3142945. PMID 17078061.
- 1 2 Adams, D. S.; Robinson, K. R.; Fukumoto, T.; Yuan, S; Albertson, R. C.; Yelick, P; Kuo, L.; McSweeney, M.; Levin, M. (2006). "Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates". Development. 133 (9): 1657–1671. doi:10.1242/dev.02341. PMC 3136117. PMID 16554361.
- 1 2 Adams, Dany S; Levin, Michael (2012). "Endogenous voltage gradients as mediators of cell-cell communication: Strategies for investigating bioelectrical signals during pattern formation". Cell and Tissue Research. 352 (1): 95–122. doi:10.1007/s00441-012-1329-4. PMC 3869965. PMID 22350846.
- ↑ Adams, D. S; Levin, M (2012). "General Principles for Measuring Resting Membrane Potential and Ion Concentration Using Fluorescent Bioelectricity Reporters". Cold Spring Harbor Protocols. 2012 (4): 385–397. doi:10.1101/pdb.top067710. PMC 4001120. PMID 22474653.
- ↑ Adams, D. S; Levin, M (2012). "Measuring Resting Membrane Potential Using the Fluorescent Voltage Reporters DiBAC4(3) and CC2-DMPE". Cold Spring Harbor Protocols. 2012 (4): 459–464. doi:10.1101/pdb.prot067702. PMC 4001116. PMID 22474652.
- ↑ Bräuner, Thomas; Hülser, Dieter F; Strasser, Reto J (1984). "Comparative measurements of membrane potentials with microelectrodes and voltage-sensitive dyes". Biochimica et Biophysica Acta (BBA) - Biomembranes. 771 (2): 208–216. doi:10.1016/0005-2736(84)90535-2. PMID 6704395.
- ↑ Deal, Parker E; Kulkarni, Rishikesh U; Al-Abdullatif, Sarah H; Miller, Evan W (2016). "Isomerically Pure Tetramethylrhodamine Voltage Reporters". Journal of the American Chemical Society. 138 (29): 9085–9088. doi:10.1021/jacs.6b05672. PMC 5222532. PMID 27428174.
- ↑ Oviedo, N. J; Nicolas, C. L; Adams, D. S; Levin, M (2008). "Live Imaging of Planarian Membrane Potential Using DiBAC4(3)". Cold Spring Harbor Protocols. 2008 (11): pdb.prot5055. doi:10.1101/pdb.prot5055. PMC 10468776. PMID 21356693.
- 1 2 3 4 5 Pai, V. P; Aw, S; Shomrat, T; Lemire, J. M; Levin, M (2011). "Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis". Development. 139 (2): 313–323. doi:10.1242/dev.073759. PMC 3243095. PMID 22159581.
- 1 2 3 Pai, Vaibhav P; Pietak, Alexis; Willocq, Valerie; Ye, Bin; Shi, Nian-Qing; Levin, Michael (2018). "HCN2 Rescues brain defects by enforcing endogenous voltage pre-patterns". Nature Communications. 9 (1): 998. Bibcode:2018NatCo...9..998P. doi:10.1038/s41467-018-03334-5. PMC 5843655. PMID 29519998.
- ↑ Pietak, Alexis; Levin, Michael (2016). "Exploring Instructive Physiological Signaling with the Bioelectric Tissue Simulation Engine". Frontiers in Bioengineering and Biotechnology. 4: 55. doi:10.3389/fbioe.2016.00055. PMC 4933718. PMID 27458581.
- ↑ Pietak, Alexis; Levin, Michael (2017). "Bioelectric gene and reaction networks: Computational modelling of genetic, biochemical and bioelectrical dynamics in pattern regulation". Journal of the Royal Society Interface. 14 (134): 20170425. doi:10.1098/rsif.2017.0425. PMC 5636277. PMID 28954851.
- 1 2 Cervera, Javier; Alcaraz, Antonio; Mafe, Salvador (2016). "Bioelectrical Signals and Ion Channels in the Modeling of Multicellular Patterns and Cancer Biophysics". Scientific Reports. 6: 20403. Bibcode:2016NatSR...620403C. doi:10.1038/srep20403. PMC 4740742. PMID 26841954.
- ↑ Cervera, Javier; Meseguer, Salvador; Mafe, Salvador (2016). "The interplay between genetic and bioelectrical signaling permits a spatial regionalisation of membrane potentials in model multicellular ensembles". Scientific Reports. 6: 35201. Bibcode:2016NatSR...635201C. doi:10.1038/srep35201. PMC 5059667. PMID 27731412.
- ↑ Cervera, Javier; Manzanares, Jose Antonio; Mafe, Salvador (2015). "Electrical Coupling in Ensembles of Nonexcitable Cells: Modeling the Spatial Map of Single Cell Potentials". The Journal of Physical Chemistry B. 119 (7): 2968–2978. doi:10.1021/jp512900x. PMID 25622192.
- ↑ Mutoh, Hiroki; Perron, Amélie; Akemann, Walther; Iwamoto, Yuka; Knöpfel, Thomas (2011). "Optogenetic monitoring of membrane potentials". Experimental Physiology. 96 (1): 13–18. doi:10.1113/expphysiol.2010.053942. PMID 20851856. S2CID 5265189.
- ↑ Pitcairn, Emily; Harris, Hannah; Epiney, Justine; Pai, Vaibhav P; Lemire, Joan M; Ye, Bin; Shi, Nian-Qing; Levin, Michael; McLaughlin, Kelly A (2017). "Coordinating heart morphogenesis: A novel role for hyperpolarization-activated cyclic nucleotide-gated (HCN) channels during cardiogenesis in Xenopus laevis". Communicative & Integrative Biology. 10 (3): e1309488. doi:10.1080/19420889.2017.1309488. PMC 5501196. PMID 28702127.
- ↑ Pai, Vaibhav P; Willocq, Valerie; Pitcairn, Emily J; Lemire, Joan M; Paré, Jean-François; Shi, Nian-Qing; McLaughlin, Kelly A; Levin, Michael (2017). "HCN4 ion channel function is required for early events that regulate anatomical left-right patterning in a nodal and lefty asymmetric gene expression-independent manner". Biology Open. 6 (10): 1445–1457. doi:10.1242/bio.025957. PMC 5665463. PMID 28818840.
- 1 2 Adams, Dany Spencer; Uzel, Sebastien G. M; Akagi, Jin; Wlodkowic, Donald; Andreeva, Viktoria; Yelick, Pamela Crotty; Devitt-Lee, Adrian; Pare, Jean-Francois; Levin, Michael (2016). "Bioelectric signalling via potassium channels: A mechanism for craniofacial dysmorphogenesis in KCNJ2-associated Andersen-Tawil Syndrome". The Journal of Physiology. 594 (12): 3245–3270. doi:10.1113/JP271930. PMC 4908029. PMID 26864374.
- ↑ Vandenberg, Laura N; Morrie, Ryan D; Adams, Dany Spencer (2011). "V-ATPase-dependent ectodermal voltage and ph regionalization are required for craniofacial morphogenesis". Developmental Dynamics. 240 (8): 1889–1904. doi:10.1002/dvdy.22685. PMC 10277013. PMID 21761475. S2CID 205768092.
- 1 2 3 Pai, V. P; Lemire, J. M; Pare, J.-F; Lin, G; Chen, Y; Levin, M (2015). "Endogenous Gradients of Resting Potential Instructively Pattern Embryonic Neural Tissue via Notch Signaling and Regulation of Proliferation". Journal of Neuroscience. 35 (10): 4366–85. doi:10.1523/JNEUROSCI.1877-14.2015. PMC 4355204. PMID 25762681.
- 1 2 Pai, Vaibhav P; Lemire, Joan M; Chen, Ying; Lin, Gufa; Levin, Michael (2015). "Local and long-range endogenous resting potential gradients antagonistically regulate apoptosis and proliferation in the embryonic CNS". The International Journal of Developmental Biology. 59 (7–8–9): 327–40. doi:10.1387/ijdb.150197ml. PMC 10505512. PMID 26198142.
- 1 2 3 Perathoner, Simon; Daane, Jacob M; Henrion, Ulrike; Seebohm, Guiscard; Higdon, Charles W; Johnson, Stephen L; Nüsslein-Volhard, Christiane; Harris, Matthew P (2014). "Bioelectric Signaling Regulates Size in Zebrafish Fins". PLOS Genetics. 10 (1): e1004080. doi:10.1371/journal.pgen.1004080. PMC 3894163. PMID 24453984.
- 1 2 Chernet, Brook T; Fields, Chris; Levin, Michael (2015). "Long-range gap junctional signaling controls oncogene-mediated tumorigenesis in Xenopus laevis embryos". Frontiers in Physiology. 5: 519. doi:10.3389/fphys.2014.00519. PMC 4298169. PMID 25646081.
- 1 2 Chernet, Brook T; Levin, Michael (2014). "Transmembrane voltage potential of somatic cells controls oncogene-mediated tumorigenesis at long-range". Oncotarget. 5 (10): 3287–306. doi:10.18632/oncotarget.1935. PMC 4102810. PMID 24830454.
- 1 2 Yoon, G; Oberoi, S; Tristani-Firouzi, M; Etheridge, S.P; Quitania, L; Kramer, J.H; Miller, B.L; Fu, Y.H; Ptáček, L.J (2006). "Andersen-Tawil syndrome: Prospective cohort analysis and expansion of the phenotype". American Journal of Medical Genetics Part A. 140A (4): 312–321. doi:10.1002/ajmg.a.31092. PMID 16419128. S2CID 33899188.
- ↑ Plaster, Nikki M; Tawil, Rabi; Tristani-Firouzi, Martin; Canún, Sonia; Bendahhou, Saı̈d; Tsunoda, Akiko; Donaldson, Matthew R; Iannaccone, Susan T; Brunt, Ewout; Barohn, Richard; Clark, John; Deymeer, Feza; George, Alfred L; Fish, Frank A; Hahn, Angelika; Nitu, Alexandru; Ozdemir, Coskun; Serdaroglu, Piraye; Subramony, S.H; Wolfe, Gil; Fu, Ying-Hui; Ptáček, Louis J (2001). "Mutations in Kir2.1 Cause the Developmental and Episodic Electrical Phenotypes of Andersen's Syndrome". Cell. 105 (4): 511–519. doi:10.1016/S0092-8674(01)00342-7. PMID 11371347. S2CID 17015195.
- 1 2 Masotti, Andrea; Uva, Paolo; Davis-Keppen, Laura; Basel-Vanagaite, Lina; Cohen, Lior; Pisaneschi, Elisa; Celluzzi, Antonella; Bencivenga, Paola; Fang, Mingyan; Tian, Mingyu; Xu, Xun; Cappa, Marco; Dallapiccola, Bruno (2015). "Keppen-Lubinsky Syndrome is Caused by Mutations in the Inwardly Rectifying K+ Channel Encoded by KCNJ6". The American Journal of Human Genetics. 96 (2): 295–300. doi:10.1016/j.ajhg.2014.12.011. PMC 4320262. PMID 25620207.
- ↑ Papoulidis, I.; Papageorgiou, E.; Siomou, E.; et al. (2014). "A patient with partial trisomy 21 and 7q deletion expresses mild Down syndrome phenotype". Gene. 536 (2): 441–443. doi:10.1016/j.gene.2013.11.078. PMID 24334122.
- ↑ Vaglio, Stefano (2010). "Volatile Signals during Pregnancy". Pheromones. Vitamins & Hormones. Vol. 83. pp. 289–304. doi:10.1016/S0083-6729(10)83012-2. ISBN 978-0-12-381516-3. PMID 20831951.
- ↑ Yamamoto, Tetsuo; Kinoshita, Manabu; Shinomiya, Nariyoshi; et al. (2010). "Pretreatment with Ascorbic Acid Prevents Lethal Gastrointestinal Syndrome in Mice Receiving a Massive Amount of Radiation". Journal of Radiation Research. 51 (2): 145–156. Bibcode:2010JRadR..51..145Y. doi:10.1269/jrr.09078. PMID 19959877.
- ↑ Capkova, Pavlina; Misovicova, Nadezda; Vrbicka, Dita (2013). "Partial trisomy and tetrasomy of chromosome 21 without down syndrome phenotype and short overview of genotype-phenotype correlation. A case report". Biomedical Papers. 158 (2): 321–325. doi:10.5507/bp.2013.077. PMID 24145769.
- ↑ Mégarbané, André; Al-Ali, Rashid; Choucair, Nancy; et al. (2016). "Temple-Baraitser Syndrome and Zimmermann-Laband Syndrome: One clinical entity?". BMC Medical Genetics. 17 (1): 42. doi:10.1186/s12881-016-0304-4. PMC 4901505. PMID 27282200.
- ↑ Mastrangelo, M.; Scheffer, I. E; Bramswig, N. C; Nair, L. D.; Myers, C. T; Dentici, M. L; Korenke, G. C; Schoch, K; Campeau, P. M.; White, S. M; Shashi, V; Kansagra, S; Van Essen, A. J; Leuzzi, V (2016). "Epilepsy in KCNH1-related syndromes". Epileptic Disorders. 18 (2): 123–136. doi:10.1684/epd.2016.0830. PMID 27267311.
- ↑ Bramswig, Nuria C; Ockeloen, C. W; Czeschik, J. C; Van Essen, A. J; Pfundt, R; Smeitink, J; Poll-The, B. T; Engels, H; Strom, T. M; Wieczorek, D; Kleefstra, T; Lüdecke, H.-J (2015). "'Splitting versus lumping': Temple–Baraitser and Zimmermann–Laband Syndromes". Human Genetics. 134 (10): 1089–1097. doi:10.1007/s00439-015-1590-1. PMID 26264464. S2CID 14238362.
- 1 2 Kortüm, Fanny; Caputo, Viviana; Bauer, Christiane K; et al. (2015). "Mutations in KCNH1 and ATP6V1B2 cause Zimmermann-Laband syndrome". Nature Genetics. 47 (6): 661–7. doi:10.1038/ng.3282. hdl:2108/118197. PMID 25915598. S2CID 12060592.
- ↑ Castori, Marco; Morlino, Silvia; Ritelli, Marco; et al. (2014). "Late diagnosis of lateral meningocele syndrome in a 55-year-old woman with symptoms of joint instability and chronic musculoskeletal pain". American Journal of Medical Genetics Part A. 164 (2): 528–534. doi:10.1002/ajmg.a.36301. PMID 24311540. S2CID 12063113.
- ↑ Perks, T; Popat, H.; Cronin, A. J.; Durning, P; Maggs, R (2013). "The orthodontic and surgical management of Zimmerman-Laband syndrome". Orthodontics. 14 (1): e168–176. doi:10.11607/ortho.897. PMID 23646327.
- ↑ Sawaki, K.; Mishima, K.; Sato, A.; et al. (2012). "Zimmermann-Laband Syndrome". Journal of Clinical Pediatric Dentistry. 36 (3): 297–300. doi:10.17796/jcpd.36.3.k854128176u764l8. PMID 22838235.
- ↑ Dufendach, K. A.; Giudicessi, J. R.; Boczek, N. J.; Ackerman, M. J. (2013). "Maternal Mosaicism Confounds the Neonatal Diagnosis of Type 1 Timothy Syndrome". Pediatrics. 131 (6): e1991–1995. doi:10.1542/peds.2012-2941. PMC 3666110. PMID 23690510.
- ↑ Splawski, Igor; Timothy, Katherine W; Sharpe, Leah M; et al. (2004). "CaV1.2 Calcium Channel Dysfunction Causes a Multisystem Disorder Including Arrhythmia and Autism". Cell. 119 (1): 19–31. doi:10.1016/j.cell.2004.09.011. PMID 15454078. S2CID 15325633.
- ↑ Margulis, Andrea V.; Mitchell, Allen A.; Gilboa, Suzanne M.; Werler, Martha M.; Mittleman, Murray A; Glynn, Robert J.; Hernandez-Diaz, Sonia (2012). "Use of topiramate in pregnancy and risk of oral clefts". American Journal of Obstetrics and Gynecology. 207 (5): 405.e1–7. doi:10.1016/j.ajog.2012.07.008. PMC 3484193. PMID 22917484.
- ↑ Hill, Denise S.; Wlodarczyk, Bogdan J.; Palacios, Ana M.; Finnell, Richard H. (2014). "Teratogenic effects of antiepileptic drugs". Expert Review of Neurotherapeutics. 10 (6): 943–959. doi:10.1586/ern.10.57. PMC 2970517. PMID 20518610.
- ↑ White, H. Steve; Smith, Misty D.; Wilcox, Karen S. (2007). "Mechanisms of Action of Antiepileptic Drugs". The Neurobiology of Epilepsy and Aging. International Review of Neurobiology. Vol. 81. pp. 85–110. doi:10.1016/S0074-7742(06)81006-8. ISBN 978-0-12-374018-2. PMID 17433919.
- ↑ Fritz, H.; Müller, D.; Hess, R. (1976). "Comparative study of the teratogenicity of phenobarbitone, diphenlhydatoin and carbamazepine in mice". Toxicology. 6 (3): 323–330. doi:10.1016/0300-483X(76)90036-6. PMID 996878.
- ↑ Feldman, Gerald L.; Weaver, D. D.; Lovrien, E. W. (1977). "The Fetal Trimethadione Syndrome". American Journal of Diseases of Children. 131 (12): 1389–1392. doi:10.1001/archpedi.1977.02120250071012. PMID 412416.
- 1 2 Barker, A. T.; Jaffe, L. F.; Vanable, J. W. (1982). "The glabrous epidermis of cavies contains a powerful battery". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 242 (3): R358–366. doi:10.1152/ajpregu.1982.242.3.R358. PMID 7065232.
- 1 2 Blüh, O; Scott, B. I. H. (1950). "Vibrating Probe Electrometer for the Measurement of Bioelectric Potentials". Review of Scientific Instruments. 21 (10): 867–868. Bibcode:1950RScI...21..867B. doi:10.1063/1.1745444. PMID 14786543.
- ↑ Chiang, Meicheng; Robinson, Kenneth R.; Vanable, Joseph W. (1992). "Electrical fields in the vicinity of epithelial wounds in the isolated bovine eye". Experimental Eye Research. 54 (6): 999–1003. doi:10.1016/0014-4835(92)90164-N. PMID 1521590.
- ↑ Chiang, Meicheng; Cragoe, Edward J; Vanable, Joseph W (1991). "Intrinsic electric fields promote epithelization of wounds in the newt, Notophthalmus viridescens". Developmental Biology. 146 (2): 377–385. doi:10.1016/0012-1606(91)90239-Y. PMID 1864462.
- 1 2 Reid, Brian; Song, Bing; McCaig, Colin D; Zhao, Min (2005). "Wound healing in rat cornea: The role of electric currents". The FASEB Journal. 19 (3): 379–386. doi:10.1096/fj.04-2325com. PMC 1459277. PMID 15746181.
- 1 2 3 4 Zhao, Min; Song, Bing; Pu, Jin; et al. (2006). "Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-γ and PTEN". Nature. 442 (7101): 457–460. Bibcode:2006Natur.442..457Z. doi:10.1038/nature04925. PMID 16871217. S2CID 4391475.
- ↑ Shen, Yunyun; Pfluger, Trisha; Ferreira, Fernando; Liang, Jiebing; Navedo, Manuel F; Zeng, Qunli; Reid, Brian; Zhao, Min (2016). "Diabetic cornea wounds produce significantly weaker electric signals that may contribute to impaired healing". Scientific Reports. 6: 26525. Bibcode:2016NatSR...626525S. doi:10.1038/srep26525. PMC 4901296. PMID 27283241.
- ↑ Maurice, D. M. The permeability to sodium ions of the living rabbit's cornea. J Physiol 112, 367-391. Pubmed Central reference number: PMC1393020
- ↑ Klyce, S. D. Electrical profiles in the corneal epithelium. J Physiol 226, 407-429. Pubmed Central reference number: PMC1331188
- ↑ Song, B (2004). "Nerve regeneration and wound healing are stimulated and directed by an endogenous electrical field in vivo". Journal of Cell Science. 117 (20): 4681–4690. doi:10.1242/jcs.01341. PMID 15371524.
- ↑ Lin, F.; Baldessari, F.; Gyenge, C. C.; et al. (2008). "Lymphocyte Electrotaxis in Vitro and in Vivo". The Journal of Immunology. 181 (4): 2465–2471. doi:10.4049/jimmunol.181.4.2465. PMC 2572691. PMID 18684937.
- ↑ Yang, H.-y; Charles, R.-P; Hummler, E; Baines, D. L.; Isseroff, R. R. (2013). "The epithelial sodium channel mediates the directionality of galvanotaxis in human keratinocytes". Journal of Cell Science. 126 (9): 1942–1951. doi:10.1242/jcs.113225. PMC 3666251. PMID 23447677.
- ↑ Allen, Greg M.; Mogilner, Alex; Theriot, Julie A. (2013). "Electrophoresis of Cellular Membrane Components Creates the Directional Cue Guiding Keratocyte Galvanotaxis". Current Biology. 23 (7): 560–568. doi:10.1016/j.cub.2013.02.047. PMC 3718648. PMID 23541731.
- ↑ Chang, Fred; Minc, Nicolas (2014). "Electrochemical Control of Cell and Tissue Polarity". Annual Review of Cell and Developmental Biology. 30: 317–336. doi:10.1146/annurev-cellbio-100913-013357. PMID 25062359.
- ↑ Robinson, K. R. (1985). "The responses of cells to electrical fields: A review". The Journal of Cell Biology. 101 (6): 2023–2037. doi:10.1083/jcb.101.6.2023. PMC 2114002. PMID 3905820.
- ↑ Nishimura, K. Y; Isseroff, R. R; Nuccitelli, R (1996). "Human keratinocytes migrate to the negative pole in direct current electric fields comparable to those measured in mammalian wounds". Journal of Cell Science. 109 (1): 199–207. doi:10.1242/jcs.109.1.199. PMID 8834804.
- ↑ Zhao, M.; Agius-Fernandez, A.; Forrester, J. V.; McCaig, C. D. (1996). "Orientation and directed migration of cultured corneal epithelial cells in small electric fields are serum dependent". Journal of Cell Science. 109 (6): 1405–1414. doi:10.1242/jcs.109.6.1405. PMID 8799828.
- ↑ Gruler, Hans; Nuccitelli, Richard (2000). "The Galvanotaxis Response Mechanism of Keratinocytes Can Be Modeled as a Proportional Controller". Cell Biochemistry and Biophysics. 33 (1): 33–51. doi:10.1385/CBB:33:1:33. PMID 11322511. S2CID 11731666.
- ↑ Zhao, M; Agius-Fernandez, A; Forrester, J. V; McCaig, C. D (1996). "Directed migration of corneal epithelial sheets in physiological electric fields". Investigative Ophthalmology & Visual Science. 37 (13): 2548–2558. PMID 8977469.
- ↑ Nakajima, Ken-Ichi; Zhu, Kan; Sun, Yao-Hui; et al. (2015). "KCNJ15/Kir4.2 couples with polyamines to sense weak extracellular electric fields in galvanotaxis". Nature Communications. 6: 8532. Bibcode:2015NatCo...6.8532N. doi:10.1038/ncomms9532. PMC 4603535. PMID 26449415.
- ↑ Gao, Runchi; Zhao, Siwei; Jiang, Xupin; et al. (2015). "A large-scale screen reveals genes that mediate electrotaxis in Dictyostelium discoideum". Science Signaling. 8 (378): ra50. doi:10.1126/scisignal.aab0562. PMC 4470479. PMID 26012633.
- ↑ Djamgoz, M. B. A; Mycielska, M; Madeja, Z; et al. (2001). "Directional movement of rat prostate cancer cells in direct-current electric field: Involvement of voltagegated Na+ channel activity". Journal of Cell Science. 114 (14): 2697–2705. doi:10.1242/jcs.114.14.2697. PMID 11683396.
- ↑ Zhang, Gaofeng; Edmundson, Mathew; Telezhkin, Vsevolod; et al. (2016). "The Role of Kv1.2 Channel in Electrotaxis Cell Migration". Journal of Cellular Physiology. 231 (6): 1375–1384. doi:10.1002/jcp.25259. PMC 4832312. PMID 26580832.
- ↑ Zhang, Gaofeng; Gu, Yu; Begum, Rumena; et al. (2016). "Kindlin-1 Regulates Keratinocyte Electrotaxis". Journal of Investigative Dermatology. 136 (11): 2229–2239. doi:10.1016/j.jid.2016.05.129. PMC 5756539. PMID 27427485.
- ↑ Zhao, MIN; Pu, JIN; Forrester, John V; et al. (2002). "Membrane lipids, EGF receptors, and intracellular signals colocalize and are polarized in epithelial cells moving directionally in a physiological electric field". The FASEB Journal. 16 (8): 857–859. doi:10.1096/fj.01-0811fje. PMID 11967227. S2CID 31682478.
- ↑ Lin, Bo-Jian; Tsao, Shun-hao; Chen, Alex; et al. (2017). "Lipid rafts sense and direct electric field-induced migration". Proceedings of the National Academy of Sciences. 114 (32): 8568–8573. Bibcode:2017PNAS..114.8568L. doi:10.1073/pnas.1702526114. PMC 5559012. PMID 28739955.
- ↑ Maden, M. (1991). A history of regeneration research. Cambridge University.
- ↑ Marsh, Gordon; Beams, H. W (1952). "Electrical control of morphogenesis in regenerating dugesia tigrina. I. Relation of axial polarity to field strength". Journal of Cellular and Comparative Physiology. 39 (2): 191–213. doi:10.1002/jcp.1030390203. PMID 14946235.
- ↑ Borgens, Richard B (1984). "Are limb development and limb regeneration both initiated by an integumentary wounding?". Differentiation. 28 (2): 87–93. doi:10.1111/j.1432-0436.1984.tb00270.x. PMID 6526168.
- ↑ Lykken, David T (1970). "Square-Wave Analysis of Skin Impedance". Psychophysiology. 7 (2): 262–275. doi:10.1111/j.1469-8986.1970.tb02232.x. PMID 5499129.
- ↑ Smith, Stephen D (1967). "Induction of partial limb regeneration in Rana pipiens by galvanic stimulation". The Anatomical Record. 158 (1): 89–97. doi:10.1002/ar.1091580110. PMID 6033441. S2CID 22547794.
- 1 2 Jenkins, Lisa S; Duerstock, Bradley S; Borgens, Richard B (1996). "Reduction of the Current of Injury Leaving the Amputation Inhibits Limb Regeneration in the Red Spotted Newt". Developmental Biology. 178 (2): 251–262. doi:10.1006/dbio.1996.0216. PMID 8812127.
- ↑ Borgens, R. B; Vanable, J. W; Jaffe, L. F (1977). "Bioelectricity and regeneration: Large currents leave the stumps of regenerating newt limbs". Proceedings of the National Academy of Sciences. 74 (10): 4528–32. Bibcode:1977PNAS...74.4528B. doi:10.1073/pnas.74.10.4528. PMC 431978. PMID 270701.
- 1 2 Borgens, Richard B; Vanable, Joseph W; Jaffe, Lionel F (1979). "Small artificial currents enhance Xenopus limb regeneration". Journal of Experimental Zoology. 207 (2): 217–226. doi:10.1002/jez.1402070206.
- ↑ McCaig, C. D. Electric Fields in Vertebrate Repair., (The Physiological Society, 1989).
- 1 2 Yasuda, Iwao (1974). "Mechanical and electrical callus". Annals of the New York Academy of Sciences. 238: 457–465. doi:10.1111/j.1749-6632.1974.tb26812.x. PMID 4531275. S2CID 84676921.
- ↑ Fukada, Eiichi; Yasuda, Iwao (1957). "On the Piezoelectric Effect of Bone". Journal of the Physical Society of Japan. 12 (10): 1158–1162. Bibcode:1957JPSJ...12.1158F. doi:10.1143/JPSJ.12.1158.
- ↑ Bruce M. Carlson, M. D., Ph.D. Principles of Regenerative Biology. (Academic Press, 2007).
- 1 2 Golding, Anne; Guay, Justin A; Herrera-Rincon, Celia; Levin, Michael; Kaplan, David L (2016). "A Tunable Silk Hydrogel Device for Studying Limb Regeneration in Adult Xenopus Laevis". PLOS ONE. 11 (6): e0155618. Bibcode:2016PLoSO..1155618G. doi:10.1371/journal.pone.0155618. PMC 4892606. PMID 27257960.
- 1 2 Hechavarria, Daniel; Dewilde, Abiche; Braunhut, Susan; Levin, Michael; Kaplan, David L (2010). "BioDome regenerative sleeve for biochemical and biophysical stimulation of tissue regeneration". Medical Engineering & Physics. 32 (9): 1065–1073. doi:10.1016/j.medengphy.2010.07.010. PMC 2967604. PMID 20708956.
- ↑ Leppik, Liudmila P; Froemel, Dara; Slavici, Andrei; Ovadia, Zachri N; Hudak, Lukasz; Henrich, Dirk; Marzi, Ingo; Barker, John H (2015). "Effects of electrical stimulation on rat limb regeneration, a new look at an old model". Scientific Reports. 5: 18353. Bibcode:2015NatSR...518353L. doi:10.1038/srep18353. PMC 4683620. PMID 26678416.
- ↑ Reid, Brian; Song, Bing; Zhao, Min (2009). "Electric currents in Xenopus tadpole tail regeneration". Developmental Biology. 335 (1): 198–207. doi:10.1016/j.ydbio.2009.08.028. PMID 19733557.
- ↑ Tseng, Aisun; Levin, Michael (2014). "Cracking the bioelectric code: Probing endogenous ionic controls of pattern formation". Communicative & Integrative Biology. 6 (1): e22595. doi:10.4161/cib.22595. PMC 3689572. PMID 23802040.
- 1 2 3 Adams, D. S; Masi, A; Levin, M (2007). "H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration". Development. 134 (7): 1323–1335. doi:10.1242/dev.02812. PMID 17329365.
- 1 2 3 4 Tseng, A.-S; Beane, W. S; Lemire, J. M; Masi, A; Levin, M (2010). "Induction of Vertebrate Regeneration by a Transient Sodium Current". Journal of Neuroscience. 30 (39): 13192–13200. doi:10.1523/JNEUROSCI.3315-10.2010. PMC 2965411. PMID 20881138.
- 1 2 3 Adams, D. S; Tseng, A.-S; Levin, M (2013). "Light-activation of the Archaerhodopsin H+-pump reverses age-dependent loss of vertebrate regeneration: Sparking system-level controls in vivo". Biology Open. 2 (3): 306–313. doi:10.1242/bio.20133665. PMC 3603412. PMID 23519324.
- ↑ Oviedo, N. J; Levin, M (2007). "Smedinx-11 is a planarian stem cell gap junction gene required for regeneration and homeostasis". Development. 134 (17): 3121–3131. doi:10.1242/dev.006635. PMID 17670787.
- ↑ Beane, W. S; Morokuma, J; Lemire, J. M; Levin, M (2012). "Bioelectric signaling regulates head and organ size during planarian regeneration". Development. 140 (2): 313–322. doi:10.1242/dev.086900. PMC 3597208. PMID 23250205.
- ↑ Beane, Wendy S; Morokuma, Junji; Adams, Dany S; Levin, Michael (2011). "A Chemical Genetics Approach Reveals H,K-ATPase-Mediated Membrane Voltage is Required for Planarian Head Regeneration". Chemistry & Biology. 18 (1): 77–89. doi:10.1016/j.chembiol.2010.11.012. PMC 3278711. PMID 21276941.
- 1 2 Emmons-Bell, Maya; Durant, Fallon; Hammelman, Jennifer; Bessonov, Nicholas; Volpert, Vitaly; Morokuma, Junji; Pinet, Kaylinnette; Adams, Dany; Pietak, Alexis; Lobo, Daniel; Levin, Michael (2015). "Gap Junctional Blockade Stochastically Induces Different Species-Specific Head Anatomies in Genetically Wild-Type Girardia dorotocephala Flatworms". International Journal of Molecular Sciences. 16 (11): 27865–27896. doi:10.3390/ijms161126065. PMC 4661923. PMID 26610482.
- ↑ Nogi, Taisaku; Levin, Michael (2005). "Characterization of innexin gene expression and functional roles of gap-junctional communication in planarian regeneration". Developmental Biology. 287 (2): 314–335. doi:10.1016/j.ydbio.2005.09.002. PMID 16243308.
- ↑ Oviedo, Néstor J; Morokuma, Junji; Walentek, Peter; Kema, Ido P; Gu, Man Bock; Ahn, Joo-Myung; Hwang, Jung Shan; Gojobori, Takashi; Levin, Michael (2010). "Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration". Developmental Biology. 339 (1): 188–199. doi:10.1016/j.ydbio.2009.12.012. PMC 2823934. PMID 20026026.
- 1 2 Durant, Fallon; Morokuma, Junji; Fields, Christopher; Williams, Katherine; Adams, Dany Spencer; Levin, Michael (2017). "Long-Term, Stochastic Editing of Regenerative Anatomy via Targeting Endogenous Bioelectric Gradients". Biophysical Journal. 112 (10): 2231–2243. Bibcode:2017BpJ...112.2231D. doi:10.1016/j.bpj.2017.04.011. PMC 5443973. PMID 28538159.
- ↑ Neuhof, Moran; Levin, Michael; Rechavi, Oded (2016). "Vertically- and horizontally-transmitted memories – the fading boundaries between regeneration and inheritance in planaria". Biology Open. 5 (9): 1177–1188. doi:10.1242/bio.020149. PMC 5051648. PMID 27565761.
- 1 2 Lobikin, Maria; Chernet, Brook; Lobo, Daniel; Levin, Michael (2012). "Resting potential, oncogene-induced tumorigenesis, and metastasis: the bioelectric basis of cancerin vivo". Physical Biology. 9 (6): 065002. Bibcode:2012PhBio...9f5002L. doi:10.1088/1478-3975/9/6/065002. PMC 3528107. PMID 23196890.
- ↑ Yang, Ming; Brackenbury, William J. (2013). "Membrane potential and cancer progression". Frontiers in Physiology. 4: 185. doi:10.3389/fphys.2013.00185. PMC 3713347. PMID 23882223.
- 1 2 Kandouz, Mustapha; Batist, Gerald (2010). "Gap junctions and connexins as therapeutic targets in cancer". Expert Opinion on Therapeutic Targets. 14 (7): 681–692. doi:10.1517/14728222.2010.487866. PMID 20446866. S2CID 30844116.
- ↑ Leithe, Edward; Sirnes, Solveig; Omori, Yasufumi; Rivedal, Edgar (2006). "Downregulation of Gap Junctions in Cancer Cells". Critical Reviews in Oncogenesis. 12 (3–4): 225–256. doi:10.1615/CritRevOncog.v12.i3-4.30. PMID 17425504.
- ↑ Trosko, J.E (2005). "The role of stem cells and gap junctions as targets for cancer chemoprevention and chemotherapy". Biomedicine & Pharmacotherapy. 59: S326–331. doi:10.1016/S0753-3322(05)80065-4. PMID 16507402.
- ↑ Pardo, Luis A; Stühmer, Walter (2013). "The roles of K+ channels in cancer". Nature Reviews Cancer. 14 (1): 39–48. doi:10.1038/nrc3635. PMID 24336491. S2CID 28497543.
- ↑ Huang, Xi; Jan, Lily Yeh (2014). "Targeting potassium channels in cancer". The Journal of Cell Biology. 206 (2): 151–162. doi:10.1083/jcb.201404136. PMC 4107787. PMID 25049269.
- ↑ Arcangeli, Annarosa; Becchetti, Andrea (2010). "New Trends in Cancer Therapy: Targeting Ion Channels and Transporters". Pharmaceuticals. 3 (4): 1202–1224. doi:10.3390/ph3041202. PMC 4034029. PMID 27713296.
- ↑ Fraser, S. P; Ozerlat-Gunduz, I; Brackenbury, W. J; Fitzgerald, E. M; Campbell, T. M; Coombes, R. C; Djamgoz, M. B. A (2014). "Regulation of voltage-gated sodium channel expression in cancer: Hormones, growth factors and auto-regulation". Philosophical Transactions of the Royal Society B: Biological Sciences. 369 (1638): 20130105. doi:10.1098/rstb.2013.0105. PMC 3917359. PMID 24493753.
- ↑ Djamgoz, M. B. A; Coombes, R. C; Schwab, A (2014). "Ion transport and cancer: From initiation to metastasis". Philosophical Transactions of the Royal Society B: Biological Sciences. 369 (1638): 20130092. doi:10.1098/rstb.2013.0092. PMC 3917347. PMID 24493741.
- ↑ Frede, Julia; Fraser, Scott P; Oskay-Özcelik, Gülten; Hong, Yeosun; Ioana Braicu, E; Sehouli, Jalid; Gabra, Hani; Djamgoz, Mustafa B.A (2013). "Ovarian cancer: Ion channel and aquaporin expression as novel targets of clinical potential". European Journal of Cancer. 49 (10): 2331–2344. doi:10.1016/j.ejca.2013.03.016. PMID 23683551.
- ↑ Yildirim, Senay; Altun, Seyhan; Gumushan, Hatice; Patel, Anup; Djamgoz, Mustafa B.A (2012). "Voltage-gated sodium channel activity promotes prostate cancer metastasis in vivo". Cancer Letters. 323 (1): 58–61. doi:10.1016/j.canlet.2012.03.036. PMID 22484465.
- ↑ Blackiston, D; Adams, D. S; Lemire, J. M; Lobikin, M; Levin, M (2010). "Transmembrane potential of Gly Cl-expressing instructor cells induces a neoplastic-like conversion of melanocytes via a serotonergic pathway". Disease Models & Mechanisms. 4 (1): 67–85. doi:10.1242/dmm.005561. PMC 3008964. PMID 20959630.
- ↑ Morokuma, J; Blackiston, D; Adams, D. S; Seebohm, G; Trimmer, B; Levin, M (2008). "Modulation of potassium channel function confers a hyperproliferative invasive phenotype on embryonic stem cells". Proceedings of the National Academy of Sciences. 105 (43): 16608–13. Bibcode:2008PNAS..10516608M. doi:10.1073/pnas.0808328105. JSTOR 25465142. PMC 2575467. PMID 18931301.
- 1 2 Chernet, Brook T; Adams, Dany S; Lobikin, Maria; Levin, Michael (2016). "Use of genetically encoded, light-gated ion translocators to control tumorigenesis". Oncotarget. 7 (15): 19575–19588. doi:10.18632/oncotarget.8036. PMC 4991402. PMID 26988909.
- ↑ Chernet, B. T; Levin, M (2013). "Transmembrane voltage potential is an essential cellular parameter for the detection and control of tumor development in a Xenopus model". Disease Models & Mechanisms. 6 (3): 595–607. doi:10.1242/dmm.010835. PMC 3634644. PMID 23471912.
- ↑ Li, Chunmei; Levin, Michael; Kaplan, David L (2016). "Bioelectric modulation of macrophage polarization". Scientific Reports. 6: 21044. Bibcode:2016NatSR...621044L. doi:10.1038/srep21044. PMC 4751571. PMID 26869018.
- ↑ Özkucur, Nurdan; Quinn, Kyle P; Pang, Jin C; Du, Chuang; Georgakoudi, Irene; Miller, Eric; Levin, Michael; Kaplan, David L (2015). "Membrane potential depolarization causes alterations in neuron arrangement and connectivity in cocultures". Brain and Behavior. 5 (1): 24–38. doi:10.1002/brb3.295. PMC 4321392. PMID 25722947.
- ↑ Lobikin, Maria; Paré, Jean-François; Kaplan, David L; Levin, Michael (2015). "Selective depolarization of transmembrane potential alters muscle patterning and muscle cell localization in Xenopus laevis embryos". The International Journal of Developmental Biology. 59 (7–8–9): 303–311. doi:10.1387/ijdb.150198ml. PMC 10461602. PMID 26198143.
- ↑ Sundelacruz, Sarah; Li, Chunmei; Choi, Young Jun; Levin, Michael; Kaplan, David L (2013). "Bioelectric modulation of wound healing in a 3D in vitro model of tissue-engineered bone". Biomaterials. 34 (28): 6695–6705. doi:10.1016/j.biomaterials.2013.05.040. PMC 3724996. PMID 23764116.
- ↑ Sundelacruz, Sarah; Levin, Michael; Kaplan, David L (2013). "Depolarization Alters Phenotype, Maintains Plasticity of Predifferentiated Mesenchymal Stem Cells". Tissue Engineering Part A. 19 (17–18): 1889–1908. doi:10.1089/ten.tea.2012.0425.rev. PMC 3726227. PMID 23738690.
- ↑ Hinard, V; Belin, D; Konig, S; Bader, C. R; Bernheim, L (2008). "Initiation of human myoblast differentiation via dephosphorylation of Kir2.1 K+ channels at tyrosine 242". Development. 135 (5): 859–867. doi:10.1242/dev.011387. PMID 18216177.
- ↑ Levin, Michael (2012). "Molecular bioelectricity in developmental biology: New tools and recent discoveries". BioEssays. 34 (3): 205–217. doi:10.1002/bies.201100136. PMC 3430077. PMID 22237730.
- ↑ Levin, Michael (2013). "Reprogramming cells and tissue patterning via bioelectrical pathways: Molecular mechanisms and biomedical opportunities". Wiley Interdisciplinary Reviews: Systems Biology and Medicine. 5 (6): 657–676. doi:10.1002/wsbm.1236. PMC 3841289. PMID 23897652.
- ↑ Mathews, Juanita; Levin, Michael (2017). "Gap junctional signaling in pattern regulation: Physiological network connectivity instructs growth and form". Developmental Neurobiology. 77 (5): 643–673. doi:10.1002/dneu.22405. PMC 10478170. PMID 27265625.
- ↑ Tseng, Ai-Sun; Levin, Michael (2012). "Transducing Bioelectric Signals into Epigenetic Pathways During Tadpole Tail Regeneration". The Anatomical Record. 295 (10): 1541–1451. doi:10.1002/ar.22495. PMC 3442154. PMID 22933452.
- ↑ Levin, Michael (2007). "Large-scale biophysics: Ion flows and regeneration". Trends in Cell Biology. 17 (6): 261–270. doi:10.1016/j.tcb.2007.04.007. PMID 17498955.
- ↑ Knopfel, T; Lin, M. Z; Levskaya, A; Tian, L; Lin, J. Y; Boyden, E. S (2010). "Toward the Second Generation of Optogenetic Tools". Journal of Neuroscience. 30 (45): 14998–5004. doi:10.1523/JNEUROSCI.4190-10.2010. PMC 2997431. PMID 21068304.
- ↑ Fenno, Lief; Yizhar, Ofer; Deisseroth, Karl (2011). "The Development and Application of Optogenetics". Annual Review of Neuroscience. 34: 389–412. doi:10.1146/annurev-neuro-061010-113817. PMC 6699620. PMID 21692661.
- ↑ Long, Xiaoyang; Ye, Jing; Zhao, Di; Zhang, Sheng-Jia (2015). "Magnetogenetics: Remote non-invasive magnetic activation of neuronal activity with a magnetoreceptor". Science Bulletin. 60 (24): 2107–2119. Bibcode:2015SciBu..60.2107L. doi:10.1007/s11434-015-0902-0. PMC 4692962. PMID 26740890.
- ↑ Wilson, Maxwell Z; Ravindran, Pavithran T; Lim, Wendell A; Toettcher, Jared E (2017). "Tracing Information Flow from Erk to Target Gene Induction Reveals Mechanisms of Dynamic and Combinatorial Control". Molecular Cell. 67 (5): 757–769.e5. doi:10.1016/j.molcel.2017.07.016. PMC 5591080. PMID 28826673.
- ↑ Bugaj, Lukasz J; o'Donoghue, Geoff P; Lim, Wendell A (2017). "Interrogating cellular perception and decision making with optogenetic tools". The Journal of Cell Biology. 216 (1): 25–28. doi:10.1083/jcb.201612094. PMC 5223619. PMID 28003330.
- ↑ Mitchell, Amir; Lim, Wendell (2016). "Cellular perception and misperception: Internal models for decision-making shaped by evolutionary experience". BioEssays. 38 (9): 845–849. doi:10.1002/bies.201600090. PMC 4996742. PMID 27461864.
- ↑ Fischbach, M. A; Bluestone, J. A; Lim, W. A (2013). "Cell-Based Therapeutics: The Next Pillar of Medicine". Science Translational Medicine. 5 (179): 179ps7. doi:10.1126/scitranslmed.3005568. PMC 3772767. PMID 23552369.
- ↑ Chau, Angela H; Walter, Jessica M; Gerardin, Jaline; Tang, Chao; Lim, Wendell A (2012). "Designing Synthetic Regulatory Networks Capable of Self-Organizing Cell Polarization". Cell. 151 (2): 320–332. doi:10.1016/j.cell.2012.08.040. PMC 3498761. PMID 23039994.
- ↑ Bashor, Caleb J; Horwitz, Andrew A; Peisajovich, Sergio G; Lim, Wendell A (2010). "Rewiring Cells: Synthetic Biology as a Tool to Interrogate the Organizational Principles of Living Systems". Annual Review of Biophysics. 39: 515–37. doi:10.1146/annurev.biophys.050708.133652. PMC 2965450. PMID 20192780.
- ↑ Pezzulo, Giovanni; Levin, Michael (2016). "Top-down models in biology: Explanation and control of complex living systems above the molecular level". Journal of the Royal Society Interface. 13 (124): 20160555. doi:10.1098/rsif.2016.0555. PMC 5134011. PMID 27807271.
- 1 2 Pezzulo, G; Levin, M (2015). "Re-membering the body: Applications of computational neuroscience to the top-down control of regeneration of limbs and other complex organs". Integrative Biology. 7 (12): 1487–1517. doi:10.1039/c5ib00221d. PMC 4667987. PMID 26571046.
- ↑ Friston, K; Levin, M; Sengupta, B; Pezzulo, G (2015). "Knowing one's place: A free-energy approach to pattern regulation". Journal of the Royal Society Interface. 12 (105): 20141383. doi:10.1098/rsif.2014.1383. PMC 4387527. PMID 25788538.
- ↑ Levin, Michael (2014). "Endogenous bioelectrical networks store non-genetic patterning information during development and regeneration". The Journal of Physiology. 592 (11): 2295–2305. doi:10.1113/jphysiol.2014.271940. PMC 4048089. PMID 24882814.
- ↑ McNamara, Harold M; Zhang, Hongkang; Werley, Christopher A; Cohen, Adam E (2016). "Optically Controlled Oscillators in an Engineered Bioelectric Tissue". Physical Review X. 6 (3): 031001. Bibcode:2016PhRvX...6c1001M. doi:10.1103/PhysRevX.6.031001.
- ↑ Rigas, S; Debrosses, G; Haralampidis, K; Vicente-Agullo, F; Feldmann, K. A; Grabov, A; Dolan, L; Hatzopoulos, P (2001). "TRH1 encodes a potassium transporter required for tip growth in Arabidopsis root hairs". The Plant Cell. 13 (1): 139–151. doi:10.1105/tpc.13.1.139. PMC 102205. PMID 11158535.
- 1 2 Dahal, G. R; Rawson, J; Gassaway, B; Kwok, B; Tong, Y; Ptácek, L. J; Bates, E (2012). "An inwardly rectifying K+ channel is required for patterning". Development. 139 (19): 3653–3664. doi:10.1242/dev.078592. PMC 3436115. PMID 22949619.
- ↑ Villanueva, S; Burgos, J.; López-Cayuqueo, K. I.; et al. (2015). "Cleft Palate, Moderate Lung Developmental Retardation and Early Postnatal Lethality in Mice Deficient in the Kir7.1 Inwardly Rectifying K+ Channel". PLOS ONE. 10 (9): e0139284. Bibcode:2015PLoSO..1039284V. doi:10.1371/journal.pone.0139284. PMC 4581704. PMID 26402555.
- ↑ Simons, M; Gault, W. J.; Gotthardt, D.; et al. (2009). "Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization". Nature Cell Biology. 11 (3): 286–294. doi:10.1038/ncb1836. PMC 2803043. PMID 19234454.
- ↑ Hermle, T; Saltukoglu, D.; Grünewald, J.; et al. (2010). "Regulation of Frizzled-dependent planar polarity signaling by a V-ATPase subunit". Current Biology. 20 (14): 1269–1276. doi:10.1016/j.cub.2010.05.057. PMID 20579879. S2CID 15407237.
- ↑ Müller, C.; Maeso, I; Wittbrodt, J.; Martínez-Morales, J. R. (2013). "The medaka mutation tintachina sheds light on the evolution of V-ATPase B subunits in vertebrates". Scientific Reports. 3: 3217. Bibcode:2013NatSR...3E3217M. doi:10.1038/srep03217. PMC 3827601. PMID 24225653.
- ↑ Borthwick, K. J.; Kandemir, N.; Topaloglu, R.; et al. (2003). "A phenocopy of CAII deficiency: A novel genetic explanation for inherited infantile osteopetrosis with distal renal tubular acidosis". Journal of Medical Genetics. 40 (2): 115–121. doi:10.1136/jmg.40.2.115. PMC 1735376. PMID 12566520.
- ↑ Aldrich, Richard W. (2015). "A new standard: A review of Handbook of Ion Channels". The Journal of General Physiology. 146 (2): 119–121. doi:10.1085/jgp.201511461. PMC 4516783. PMID 26216856.
- ↑ Duque, A.; Gazula, V. R.; Kaczmarek, L. K. (2013). "Expression of Kv1.3 potassium channels regulates density of cortical interneurons". Developmental Neurobiology. 73 (11): 841–855. doi:10.1002/dneu.22105. PMC 3829632. PMID 23821603.
- ↑ Zheng, J. a. T., M. C. Handbook of ion channels. (CRC Press, 2015).
- ↑ Christensen, A. H; Chatelain, F. C.; Huttner, I. G.; et al. (2016). "The two-pore domain potassium channel, TWIK-1, has a role in the regulation of heart rate and atrial size". Journal of Molecular and Cellular Cardiology. 97: 24–35. doi:10.1016/j.yjmcc.2016.04.006. PMID 27103460.
- ↑ Simons, C; Rash, L. D.; Crawford, J; et al. (2015). "Mutations in the voltage-gated potassium channel gene KCNH1 cause Temple-Baraitser syndrome and epilepsy". Nature Genetics. 47 (1): 73–77. doi:10.1038/ng.3153. PMID 25420144. S2CID 52799681.
- ↑ Labonne, J. D.; Graves, T. D.; Shen, Y.; et al. (2016). "A microdeletion at Xq22.2 implicates a glycine receptor GLRA4 involved in intellectual disability, behavioral problems and craniofacial anomalies". BMC Neurology. 16: 132. doi:10.1186/s12883-016-0642-z. PMC 4979147. PMID 27506666.
- ↑ Hiraki, Y.; Miyatake, S.; Hayashidani, M.; et al. (2014). "Aortic aneurysm and craniosynostosis in a family with Cantu syndrome". American Journal of Medical Genetics Part A. 164A (1): 231–236. doi:10.1002/ajmg.a.36228. PMID 24352916. S2CID 73121.
- ↑ Cooper, P. E; Reutter, H.; Woelfle, J.; et al. (2014). "Cantú syndrome resulting from activating mutation in the KCNJ8 gene". Human Mutation. 35 (7): 809–813. doi:10.1002/humu.22555. PMC 4277879. PMID 24700710.
- ↑ Brownstein, C. A.; Towne, M. C.; Luquette, L. J; et al. (2013). "Mutation of KCNJ8 in a patient with Cantú syndrome with unique vascular abnormalities - support for the role of K(ATP) channels in this condition". European Journal of Medical Genetics. 56 (12): 678–682. doi:10.1016/j.ejmg.2013.09.009. PMC 3902017. PMID 24176758.
- ↑ Chong, J. X.; McMillin, M. J.; Shively, K. M.; et al. (2015). "De novo mutations in NALCN cause a syndrome characterized by congenital contractures of the limbs and face, hypotonia, and developmental delay". The American Journal of Human Genetics. 96 (3): 462–473. doi:10.1016/j.ajhg.2015.01.003. PMC 4375444. PMID 25683120.
- ↑ Uzun, S; Gökçe, S.; Wagner, K. (2005). "Cystic fibrosis transmembrane conductance regulator gene mutations in infertile males with congenital bilateral absence of the vas deferens". The Tohoku Journal of Experimental Medicine. 207 (4): 279–285. doi:10.1620/tjem.207.279. PMID 16272798.
- ↑ Wilschanski, M.; Dupuis, A.; Ellis, L.; et al. (2006). "Mutations in the cystic fibrosis transmembrane regulator gene and in vivo transepithelial potentials". American Journal of Respiratory and Critical Care Medicine. 174 (7): 787–794. doi:10.1164/rccm.200509-1377OC. PMC 2648063. PMID 16840743.
- ↑ Poirier, K.; Viot, G.; Lombardi, L.; Jauny, C.; Billuart, P.; Bienvenu, T. (2017). "Loss of Function of KCNC1 is associated with intellectual disability without seizures". European Journal of Human Genetics. 25 (5): 560–564. doi:10.1038/ejhg.2017.3. PMC 5437909. PMID 28145425.
- ↑ Veale, E. L; Hassan, M.; Walsh, Y.; et al. (2014). "Recovery of current through mutated TASK3 potassium channels underlying Birk Barel syndrome". Molecular Pharmacology. 85 (3): 397–407. doi:10.1124/mol.113.090530. PMID 24342771. S2CID 14790826.
- ↑ Barel, O; Shalev, S. A.; Ofir, R.; et al. (2008). "Maternally inherited Birk Barel mental retardation dysmorphism syndrome caused by a mutation in the genomically imprinted potassium channel KCNK9". The American Journal of Human Genetics. 83 (2): 193–199. doi:10.1016/j.ajhg.2008.07.010. PMC 2495061. PMID 18678320.
- 1 2 Gloyn, Anna L; Pearson, Ewan R; Antcliff, Jennifer F.; et al. (2004). "Activating Mutations in the Gene Encoding the ATP-Sensitive Potassium-Channel Subunit Kir6.2 and Permanent Neonatal Diabetes" (PDF). New England Journal of Medicine. 350 (18): 1838–1849. doi:10.1056/NEJMoa032922. PMID 15115830.
- ↑ Lee, M. P.; Ravenel, J. D.; Hu, R. J.; et al. (2000). "Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice". Journal of Clinical Investigation. 106 (12): 1447–1455. doi:10.1172/JCI10897. PMC 387258. PMID 11120752.
- 1 2 Weksberg, R; Nishikawa, J.; Caluseriu, O.; et al. (2001). "Tumor development in the Beckwith-Wiedemann syndrome is associated with a variety of constitutional molecular 11p15 alterations including imprinting defects of KCNQ1OT1". Human Molecular Genetics. 10 (26): 2989–3000. doi:10.1093/hmg/10.26.2989. PMID 11751681.
- ↑ Moore, E. S.; Ward, R. E; Escobar, L. F.; Carlin, M. E. (2000). "Heterogeneity in Wiedemann-Beckwith syndrome: Anthropometric evidence". American Journal of Medical Genetics. 90 (4): 283–290. doi:10.1002/(SICI)1096-8628(20000214)90:4<283::AID-AJMG4>3.0.CO;2-F. PMID 10710224.
- ↑ Wen, H.; Weiger, T. M.; Ferguson, T. S.; et al. (2005). "A Drosophila KCNQ channel essential for early embryonic development". Journal of Neuroscience. 25 (44): 10147–10156. doi:10.1523/JNEUROSCI.3086-05.2005. PMC 6725806. PMID 16267222.
- ↑ Rivas, A; Francis, H. W (2005). "Inner ear abnormalities in a Kcnq1 (Kvlqt1) knockout mouse: A model of Jervell and Lange-Nielsen syndrome". Otology & Neurotology. 26 (3): 415–424. doi:10.1097/01.mao.0000169764.00798.84. PMID 15891643. S2CID 1700736.
- ↑ Casimiro, M. C; Knollmann, B. C; Yamoah, E. N; Nie, L; Vary Jr, J. C; Sirenko, S. G; Greene, A. E; Grinberg, A; Huang, S. P; Ebert, S. N; Pfeifer, K (2004). "Targeted point mutagenesis of mouse Kcnq1: Phenotypic analysis of mice with point mutations that cause Romano-Ward syndrome in humans". Genomics. 84 (3): 555–564. doi:10.1016/j.ygeno.2004.06.007. PMID 15498462.
- ↑ Chouabe, C; Neyroud, N; Guicheney, P; et al. (1997). "Properties of KvLQT1 K+ channel mutations in Romano-Ward and Jervell and Lange-Nielsen inherited cardiac arrhythmias". The EMBO Journal. 16 (17): 5472–5479. doi:10.1093/emboj/16.17.5472. PMC 1170178. PMID 9312006.
- ↑ Bendahhou, S; Donaldson, M. R; Plaster, N. M; et al. (2003). "Defective potassium channel Kir2.1 trafficking underlies Andersen-Tawil syndrome". Journal of Biological Chemistry. 278 (51): 51779–51785. doi:10.1074/jbc.M310278200. PMID 14522976.
- ↑ Culiat, C. T .; Stubbs, L. J.; Woychik, R. P; Russell, L. B.; Johnson, D. K.; Rinchik, E. M. (1995). "Deficiency of the beta 3 subunit of the type a gamma-aminobutyric acid receptor causes cleft palate in mice". Nature Genetics. 11 (3): 344–346. doi:10.1038/ng1195-344. PMID 7581464. S2CID 19397785.
- ↑ Wee, E. L; Zimmerman, E. F (1985). "GABA uptake in embryonic palate mesenchymal cells of two mouse strains". Neurochemical Research. 10 (12): 1673–1688. doi:10.1007/bf00988609. PMID 4088436. S2CID 26049392.
- ↑ Homanics, G. E; Delorey, T. M.; Firestone, L. L.; et al. (1997). "Mice devoid of gamma-aminobutyrate type a receptor beta3 subunit have epilepsy, cleft palate, and hypersensitive behavior". Proceedings of the National Academy of Sciences of the United States of America. 94 (8): 4143–4148. Bibcode:1997PNAS...94.4143H. doi:10.1073/pnas.94.8.4143. PMC 20582. PMID 9108119.
- ↑ Rock, J. R.; Futtner, C. R.; Harfe, B. D. (2008). "The transmembrane protein TMEM16A is required for normal development of the murine trachea". Developmental Biology. 321 (1): 141–149. doi:10.1016/j.ydbio.2008.06.009. PMID 18585372.
- ↑ Rakic, P; Sidman, R. L. (1973). "Sequence of developmental abnormalities leading to granule cell deficit in cerebellar cortex of weaver mutant mice". The Journal of Comparative Neurology. 152 (2): 103–132. doi:10.1002/cne.901520202. PMID 4128371. S2CID 6553698.
- ↑ Rakic, P; Sidman, R. L (1973). "Weaver mutant mouse cerebellum: Defective neuronal migration secondary to abnormality of Bergmann glia". Proceedings of the National Academy of Sciences of the United States of America. 70 (1): 240–244. Bibcode:1973PNAS...70..240R. doi:10.1073/pnas.70.1.240. PMC 433223. PMID 4509657.
- ↑ Hatten, M. E; Liem, R. K; Mason, C. A (1986). "Weaver mouse cerebellar granule neurons fail to migrate on wild-type astroglial processes in vitro". The Journal of Neuroscience. 6 (9): 2676–2683. doi:10.1523/jneurosci.06-09-02676.1986. PMC 6568692. PMID 3528411.
- ↑ Patil, N; Cox, D. R; Bhat, D; Faham, M; Myers, R. M; Peterson, A. S (1995). "A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation". Nature Genetics. 11 (2): 126–129. doi:10.1038/ng1095-126. PMID 7550338. S2CID 23470275.
- ↑ Teng, G. Q.; Zhao, X.; Lees-Miller, J. P.; Quinn, F. R.; Li, P.; Rancourt, D. E.; London, B.; Cross, J. C.; Duff, H. J. (2008). "Homozygous missense N629D hERG (KCNH2) potassium channel mutation causes developmental defects in the right ventricle and its outflow tract and embryonic lethality". Circulation Research. 103 (12): 1483–1491. doi:10.1161/CIRCRESAHA.108.177055. PMC 2774899. PMID 18948620.
- 1 2 Than, B. L; Goos, J. A.; Sarver, A. L.; et al. (2014). "The role of KCNQ1 in mouse and human gastrointestinal cancers". Oncogene. 33 (29): 3861–3868. doi:10.1038/onc.2013.350. PMC 3935979. PMID 23975432.
- ↑ Monteiro, J; Aires, R; Becker, J. D; Jacinto, A; Certal, A. C; Rodríguez-León, J (2014). "V-ATPase proton pumping activity is required for adult zebrafish appendage regeneration". PLOS ONE. 9 (3): e92594. Bibcode:2014PLoSO...992594M. doi:10.1371/journal.pone.0092594. PMC 3966808. PMID 24671205.
- ↑ Levin, M; Thorlin, T; Robinson, K. R; Nogi, T; Mercola, M (2002). "Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning". Cell. 111 (1): 77–89. doi:10.1016/s0092-8674(02)00939-x. PMID 12372302. S2CID 2502945.
- ↑ Duboc, V; Röttinger, E; Lapraz, F; et al. (2005). "Left-right asymmetry in the sea urchin embryo is regulated by nodal signaling on the right side". Developmental Cell. 9 (1): 147–158. doi:10.1016/j.devcel.2005.05.008. PMID 15992548.
- ↑ Iwashita, M; Watanabe, M; Ishii, M; et al. (2006). "Pigment pattern in jaguar/obelix zebrafish is caused by a Kir7.1 mutation: Implications for the regulation of melanosome movement". PLOS Genetics. 2 (11): e197. doi:10.1371/journal.pgen.0020197. PMC 1657052. PMID 17121467.
- ↑ Tur, J; Chapalamadugu, K. C; Padawer, T; Badole, S. L; Kilfoil Pj, 2nd; Bhatnagar, A; Tipparaju, S. M (2016). "Deletion of Kvβ1.1 subunit leads to electrical and haemodynamic changes causing cardiac hypertrophy in female murine hearts". Experimental Physiology. 101 (4): 494–508. doi:10.1113/EP085405. PMC 4827621. PMID 27038296.
- ↑ Chopra, S. S; Stroud, D. M; Watanabe, H; Bennett, J. S; Burns, C. G; Wells, K. S; Yang, T; Zhong, T. P; Roden, D. M (2010). "Voltage-gated sodium channels are required for heart development in zebrafish". Circulation Research. 106 (8): 1342–1350. doi:10.1161/CIRCRESAHA.109.213132. PMC 2869449. PMID 20339120.
- ↑ Shu, X; Cheng, K; Patel, N; et al. (2003). "Na,K-ATPase is essential for embryonic heart development in the zebrafish". Development. 130 (25): 6165–6173. doi:10.1242/dev.00844. PMID 14602677.
- ↑ Khare, S; Nick, J. A; Zhang, Y; et al. (2017). "A KCNC3 mutation causes a neurodevelopmental, non-progressive SCA13 subtype associated with dominant negative effects and aberrant EGFR trafficking". PLOS ONE. 12 (5): e0173565. Bibcode:2017PLoSO..1273565K. doi:10.1371/journal.pone.0173565. PMC 5414954. PMID 28467418.
- ↑ Starich, T. A.; Hall, D. H.; Greenstein, D. (2014). "Two classes of gap junction channels mediate soma-germline interactions essential for germline proliferation and gametogenesis in Caenorhabditis elegans". Genetics. 198 (3): 1127–1153. doi:10.1534/genetics.114.168815. PMC 4224157. PMID 25195067.
- ↑ Bauer, R; Lehmann, C; Martini, J; Eckardt, F; Hoch, M (2004). "Gap junction channel protein innexin 2 is essential for epithelial morphogenesis in the Drosophila embryo". Molecular Biology of the Cell. 15 (6): 2992–3004. doi:10.1091/mbc.E04-01-0056. PMC 420120. PMID 15047872.
- ↑ Bauer, R; Lehmann, C; Fuss, B; et al. (2002). "The Drosophila gap junction channel gene innexin 2 controls foregut development in response to Wingless signalling". Journal of Cell Science. 115 (Pt 9): 1859–1867. doi:10.1242/jcs.115.9.1859. PMID 11956317.
- ↑ Richard, M.; Hoch, M (2015). "Drosophila eye size is determined by Innexin 2-dependent Decapentaplegic signalling". Developmental Biology. 408 (1): 26–40. doi:10.1016/j.ydbio.2015.10.011. PMID 26455410.
- ↑ Debeer, P; Van Esch, H; Huysmans, C; Pijkels, E; De Smet, L; Van De Ven, W; Devriendt, K; Fryns, J. P (2005). "Novel GJA1 mutations in patients with oculo-dento-digital dysplasia (ODDD)". European Journal of Medical Genetics. 48 (4): 377–387. doi:10.1016/j.ejmg.2005.05.003. PMID 16378922.
- ↑ Pizzuti, A; Flex, E; Mingarelli, R; Salpietro, C; Zelante, L; Dallapiccola, B (2004). "A homozygous GJA1 gene mutation causes a Hallermann-Streiff/ODDD spectrum phenotype". Human Mutation. 23 (3): 286. doi:10.1002/humu.9220. PMID 14974090. S2CID 13345970.
- ↑ Ewart, J. L; Cohen, M. F; Meyer, R. A; Huang, G. Y; Wessels, A; Gourdie, R. G; Chin, A. J; Park, S. M; Lazatin, B. O; Villabon, S; Lo, C. W (1997). "Heart and neural tube defects in transgenic mice overexpressing the Cx43 gap junction gene". Development. 124 (7): 1281–1292. doi:10.1242/dev.124.7.1281. PMID 9118799. S2CID 189036.
- ↑ Reaume, A. G; De Sousa, P. A; Kulkarni, S; Langille, B. L; Zhu, D; Davies, T. C; Juneja, S. C; Kidder, G. M; Rossant, J (1995). "Cardiac malformation in neonatal mice lacking connexin43". Science. 267 (5205): 1831–1834. Bibcode:1995Sci...267.1831R. doi:10.1126/science.7892609. PMID 7892609.
- ↑ Britz-Cunningham, S. H; Shah, M. M; Zuppan, C. W; Fletcher, W. H (1995). "Mutations of the Connexin43 gap-junction gene in patients with heart malformations and defects of laterality". New England Journal of Medicine. 332 (20): 1323–1329. doi:10.1056/NEJM199505183322002. PMID 7715640.
- ↑ Civitelli, R (2008). "Cell-cell communication in the osteoblast/osteocyte lineage". Archives of Biochemistry and Biophysics. 473 (2): 188–192. doi:10.1016/j.abb.2008.04.005. PMC 2441851. PMID 18424255.
- ↑ Levin, M; Mercola, M (1999). "Gap junction-mediated transfer of left-right patterning signals in the early chick blastoderm is upstream of Shh asymmetry in the node". Development. 126 (21): 4703–4714. doi:10.1242/dev.126.21.4703. PMID 10518488.
- ↑ Becker, D. L; McGonnell, I; Makarenkova, H. P; Patel, K; Tickle, C; Lorimer, J; Green, C. R (1999). "Roles for alpha 1 connexin in morphogenesis of chick embryos revealed using a novel antisense approach". Developmental Genetics. 24 (1–2): 33–42. doi:10.1002/(SICI)1520-6408(1999)24:1/2<33::AID-DVG5>3.0.CO;2-F. PMID 10079509.
- ↑ Lecanda, F; Warlow, P. M; Sheikh, S; Furlan, F; Steinberg, T. H; Civitelli, R (2000). "Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction". The Journal of Cell Biology. 151 (4): 931–944. doi:10.1083/jcb.151.4.931. PMC 2169447. PMID 11076975.
- ↑ Araya, R; Eckardt, D; Riquelme, M. A; Willecke, K; Sáez, J. C (2003). "Presence and importance of connexin43 during myogenesis". Cell Communication & Adhesion. 10 (4–6): 451–456. doi:10.1080/cac.10.4-6.451.456. hdl:10533/174413. PMID 14681056. S2CID 33491307.
- ↑ Kanady, J. D; Dellinger, M. T; Munger, S. J; Witte, M. H; Simon, A. M (2011). "Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax". Developmental Biology. 354 (2): 253–266. doi:10.1016/j.ydbio.2011.04.004. PMC 3134316. PMID 21515254.
- ↑ Kanady, J. D; Munger, S. J; Witte, M. H; Simon, A. M (2015). "Combining Foxc2 and Connexin37 deletions in mice leads to severe defects in lymphatic vascular growth and remodeling". Developmental Biology. 405 (1): 33–46. doi:10.1016/j.ydbio.2015.06.004. PMC 4529811. PMID 26079578.
- ↑ Kumai, M; Nishii, K; Nakamura, K; Takeda, N; Suzuki, M; Shibata, Y (2000). "Loss of connexin45 causes a cushion defect in early cardiogenesis". Development. 127 (16): 3501–3512. doi:10.1242/dev.127.16.3501. PMID 10903175.
- ↑ Nishii, K; Kumai, M; Shibata, Y (2001). "Regulation of the epithelial-mesenchymal transformation through gap junction channels in heart development". Trends in Cardiovascular Medicine. 11 (6): 213–218. doi:10.1016/s1050-1738(01)00103-7. PMID 11673050.
- ↑ White, T. W (2002). "Unique and redundant connexin contributions to lens development". Science. 295 (5553): 319–320. Bibcode:2002Sci...295..319W. doi:10.1126/science.1067582. PMID 11786642. S2CID 25744002.
- ↑ Chang, Q; Tang, W; Kim, Y; Lin, X (2015). "Timed conditional null of connexin26 in mice reveals temporary requirements of connexin26 in key cochlear developmental events before the onset of hearing". Neurobiology of Disease. 73: 418–427. doi:10.1016/j.nbd.2014.09.005. PMID 25251605. S2CID 207068577.
- ↑ Watanabe, M; Iwashita, M; Ishii, M; Kurachi, Y; Kawakami, A; Kondo, S; Okada, N (2006). "Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene". EMBO Reports. 7 (9): 893–897. doi:10.1038/sj.embor.7400757. PMC 1559663. PMID 16845369.
- ↑ Iovine, M. K; Higgins, E. P; Hindes, A; Coblitz, B; Johnson, S. L (2005). "Mutations in connexin43 (GJA1) perturb bone growth in zebrafish fins". Developmental Biology. 278 (1): 208–219. doi:10.1016/j.ydbio.2004.11.005. PMID 15649473.
- ↑ Davy, A; Bush, J. O; Soriano, P (2006). "Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome". PLOS Biology. 4 (10): e315. doi:10.1371/journal.pbio.0040315. PMC 1563491. PMID 16968134.
- ↑ Sims Jr, K; Eble, D. M; Iovine, M. K (2009). "Connexin43 regulates joint location in zebrafish fins". Developmental Biology. 327 (2): 410–418. doi:10.1016/j.ydbio.2008.12.027. PMC 2913275. PMID 19150347.
- ↑ Hoptak-Solga, A. D; Nielsen, S; Jain, I; Thummel, R; Hyde, D. R; Iovine, M. K (2008). "Connexin43 (GJA1) is required in the population of dividing cells during fin regeneration". Developmental Biology. 317 (2): 541–548. doi:10.1016/j.ydbio.2008.02.051. PMC 2429987. PMID 18406403.
- ↑ Smendziuk, C. M; Messenberg, A; Vogl, A. W; Tanentzapf, G (2015). "Bi-directional gap junction-mediated soma-germline communication is essential for spermatogenesis". Development. 142 (15): 2598–2609. doi:10.1242/dev.123448. PMC 6514411. PMID 26116660.
- ↑ Oh, S. K; Shin, J. O; Baek, J. I; Lee, J; Bae, J. W; Ankamerddy, H; Kim, M. J; Huh, T. L; Ryoo, Z. Y; Kim, U. K; Bok, J; Lee, K. Y (2015). "Pannexin 3 is required for normal progression of skeletal development in vertebrates". The FASEB Journal. 29 (11): 4473–4484. doi:10.1096/fj.15-273722. PMID 26183770. S2CID 8219978.
- ↑ Onkal, R; Djamgoz, M. B (2009). "Molecular pharmacology of voltage-gated sodium channel expression in metastatic disease: Clinical potential of neonatal Nav1.5 in breast cancer". European Journal of Pharmacology. 625 (1–3): 206–219. doi:10.1016/j.ejphar.2009.08.040. PMID 19835862.
- 1 2 House, C. D; Vaske, C. J; Schwartz, A. M; Obias, V; Frank, B; Luu, T; Sarvazyan, N; Irby, R; Strausberg, R. L; Hales, T. G; Stuart, J. M; Lee, N. H (2010). "Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion". Cancer Research. 70 (17): 6957–6967. doi:10.1158/0008-5472.CAN-10-1169. PMC 2936697. PMID 20651255.
- ↑ Perez-Neut, M; Rao, V. R; Gentile, S (2016). "HERG1/Kv11.1 activation stimulates transcription of p21waf/cip in breast cancer cells via a calcineurin-dependent mechanism". Oncotarget. 7 (37): 58893–58902. doi:10.18632/oncotarget.3797. PMC 5312283. PMID 25945833.
- ↑ Lansu, K; Gentile, S (2013). "Potassium channel activation inhibits proliferation of breast cancer cells by activating a senescence program". Cell Death & Disease. 4 (6): e652. doi:10.1038/cddis.2013.174. PMC 3698542. PMID 23744352.
- ↑ Pei, L; Wiser, O; Slavin, A; Mu, D; Powers, S; Jan, L. Y; Hoey, T (2003). "Oncogenic potential of TASK3 (Kcnk9) depends on K+ channel function". Proceedings of the National Academy of Sciences. 100 (13): 7803–7807. Bibcode:2003PNAS..100.7803P. doi:10.1073/pnas.1232448100. PMC 164668. PMID 12782791.
- ↑ Saito, Tsuyoshi; Schlegel, Richard; Andresson, Thirkell; Yuge, Louis; Yamamoto, Masao; Yamasaki, Hiroshi (1998). "Induction of cell transformation by mutated 16K vacuolar H+-atpase (ductin) is accompanied by down-regulation of gap junctional intercellular communication and translocation of connexin 43 in NIH3T3 cells". Oncogene. 17 (13): 1673–1680. doi:10.1038/sj.onc.1202092. PMID 9796696.
- ↑ Gupta, N; Martin, P. M; Prasad, P. D; Ganapathy, V (2006). "SLC5A8 (SMCT1)-mediated transport of butyrate forms the basis for the tumor suppressive function of the transporter". Life Sciences. 78 (21): 2419–2425. doi:10.1016/j.lfs.2005.10.028. PMID 16375929.
- ↑ Roepke, T. K; Purtell, K; King, E. C; La Perle, K. M; Lerner, D. J; Abbott, G. W (2010). "Targeted deletion of Kcne2 causes gastritis cystica profunda and gastric neoplasia". PLOS ONE. 5 (7): e11451. Bibcode:2010PLoSO...511451R. doi:10.1371/journal.pone.0011451. PMC 2897890. PMID 20625512.
- ↑ Lee, M. P; Hu, R. J; Johnson, L. A; et al. (1997). "Human KVLQT1 gene shows tissue-specific imprinting and encompasses Beckwith-Wiedemann syndrome chromosomal rearrangements". Nature Genetics. 15 (2): 181–185. doi:10.1038/ng0297-181. PMID 9020845. S2CID 24715509.
- ↑ Martino, J. J; Wall, B. A; Mastrantoni, E; Wilimczyk, B. J; La Cava, S. N; Degenhardt, K; White, E; Chen, S (2013). "Metabotropic glutamate receptor 1 (Grm1) is an oncogene in epithelial cells". Oncogene. 32 (37): 4366–4376. doi:10.1038/onc.2012.471. PMC 3910169. PMID 23085756.
- ↑ Speyer, C. L; Smith, J. S; Banda, M; et al. (2012). "Metabotropic glutamate receptor-1: A potential therapeutic target for the treatment of breast cancer". Breast Cancer Research and Treatment. 132 (2): 565–573. doi:10.1007/s10549-011-1624-x. PMC 3898178. PMID 21681448.
- ↑ Zhang, J. T; Jiang, X. H; Xie, C; Cheng, H; Da Dong, J; Wang, Y; Fok, K. L; Zhang, X. H; Sun, T. T; Tsang, L. L; Chen, H; Sun, X. J; Chung, Y. W; Cai, Z. M; Jiang, W. G; Chan, H. C (2013). "Downregulation of CFTR promotes epithelial-to-mesenchymal transition and is associated with poor prognosis of breast cancer". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1833 (12): 2961–2969. doi:10.1016/j.bbamcr.2013.07.021. PMID 23916755.
- ↑ Xie, C; Jiang, X. H; Zhang, J. T; et al. (2013). "CFTR suppresses tumor progression through miR-193b targeting urokinase plasminogen activator (uPA) in prostate cancer". Oncogene. 32 (18): 2282–2291, 2291.e1–7. doi:10.1038/onc.2012.251. PMID 22797075. S2CID 21255355.
- ↑ Sirnes, S.; Bruun, J.; Kolberg, M.; et al. (2012). "Connexin43 acts as a colorectal cancer tumor suppressor and predicts disease outcome". International Journal of Cancer. 131 (3): 570–581. doi:10.1002/ijc.26392. PMID 21866551. S2CID 6293505.
- ↑ Schickling, B. M; England, S. K; Aykin-Burns, N; et al. (2015). "BKCa channel inhibitor modulates the tumorigenic ability of hormone-independent breast cancer cells via the Wnt pathway". Oncology Reports. 33 (2): 533–538. doi:10.3892/or.2014.3617. PMC 4306270. PMID 25422049.
- ↑ Felder, C. C.; MacArthur, L; Ma, A. L; et al. (1993). "Tumor-suppressor function of muscarinic acetylcholine receptors is associated with activation of receptor-operated calcium influx". Proceedings of the National Academy of Sciences of the United States of America. 90 (5): 1706–1710. Bibcode:1993PNAS...90.1706F. doi:10.1073/pnas.90.5.1706. PMC 45948. PMID 7680475.
- ↑ Rezania, S; Kammerer, S; Li, C; Steinecker-Frohnwieser, B; Gorischek, A; Devaney, T. T; Verheyen, S; Passegger, C. A; Tabrizi-Wizsy, N. G; Hackl, H; Platzer, D; Zarnani, A. H; Malle, E; Jahn, S. W; Bauernhofer, T; Schreibmayer, W (2016). "Overexpression of KCNJ3 gene splice variants affects vital parameters of the malignant breast cancer cell line MCF-7 in an opposing manner". BMC Cancer. 16: 628. doi:10.1186/s12885-016-2664-8. PMC 4983040. PMID 27519272.
- ↑ Kammerer, S; Sokolowski, A; Hackl, H; et al. (2016). "KCNJ3 is a new independent prognostic marker for estrogen receptor positive breast cancer patients". Oncotarget. 7 (51): 84705–84717. doi:10.18632/oncotarget.13224. PMC 5356693. PMID 27835900.
External links
 Quotations related to Developmental bioelectricity at Wikiquote
Quotations related to Developmental bioelectricity at Wikiquote