C16 (drug)
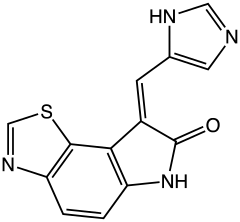 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.211.648 |
| Chemical and physical data | |
| Formula | C13H8N4OS |
| Molar mass | 268.29 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
C16 (PKRi, GW 506033X) is a drug which acts as a selective inhibitor of the enzyme double-stranded RNA-dependent protein kinase (PKR). It has been shown to effectively inhibit PKR function in vivo and has neuroprotective and nootropic effects in animal studies.[1][2][3][4][5][6][7][8]
See also
References
- ↑ Jammi, NV; Whitby, LR; Beal, PA (Aug 2003). "Small molecule inhibitors of the RNA-dependent protein kinase". Biochemical and Biophysical Research Communications. 308 (1): 50–7. doi:10.1016/s0006-291x(03)01318-4. PMID 12890478.
- ↑ Shimazawa, M; Hara, H (Dec 2006). "Inhibitor of double stranded RNA-dependent protein kinase protects against cell damage induced by ER stress". Neuroscience Letters. 409 (3): 192–5. doi:10.1016/j.neulet.2006.09.074. PMID 17055645. S2CID 43133290.
- ↑ Ingrand, S.; Barrier, L.; Lafay-Chebassier, C.; Fauconneau, B.; Page, G. N.; Hugon, J. (2007). "The oxindole/imidazole derivative C16 reduces in vivo brain PKR activation". FEBS Letters. 581 (23): 4473–4478. doi:10.1016/j.febslet.2007.08.022. PMID 17761171.
- ↑ Chen, H. M.; Wang, L.; d'Mello, S. R. (2008). "A chemical compound commonly used to inhibit PKR, {8-(imidazol-4-ylmethylene)-6H-azolidino[5,4-g] benzothiazol-7-one}, protects neurons by inhibiting cyclin-dependent kinase". European Journal of Neuroscience. 28 (10): 2003–2016. doi:10.1111/j.1460-9568.2008.06491.x. PMC 3320856. PMID 19046382.
- ↑ Couturier, J; Morel, M; Pontcharraud, R; Gontier, V; Fauconneau, B; Paccalin, M; Page, G (Jan 2010). "Interaction of double-stranded RNA-dependent protein kinase (PKR) with the death receptor signaling pathway in amyloid beta (Abeta)-treated cells and in APPSLPS1 knock-in mice". Journal of Biological Chemistry. 285 (2): 1272–82. doi:10.1074/jbc.M109.041954. PMC 2801255. PMID 19889624.
- ↑ Zhu PJ, Huang W, Kalikulov D, Yoo JW, Placzek AN, Stoica L, Zhou H, Bell JC, Friedlander MJ, Krnjevic K, Noebels JL, Costa-Mattioli M (2011). "Suppression of PKR Promotes Network Excitability and Enhanced Cognition by Interferon-γ-Mediated Disinhibition". Cell. 147 (6): 1384–1396. doi:10.1016/j.cell.2011.11.029. PMC 3569515. PMID 22153080.
- ↑ Hwang KD, Bak MS, Kim SJ, Rhee S, Lee YS. Restoring synaptic plasticity and memory in mouse models of Alzheimer's disease by PKR inhibition. Mol Brain. 2017;10(1):57. doi:10.1186/s13041-017-0338-3
- ↑ Gal-Ben-Ari S, Barrera I, Ehrlich M, Rosenblum K. PKR: A Kinase to Remember. Front Mol Neurosci. 2019;11:480. doi:10.3389/fnmol.2018.00480
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.