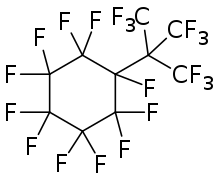
Perfluoro tert-butylcyclohexane
 | |
| Clinical data | |
|---|---|
| Trade names | Oxycyte |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.245.872 |
| Chemical and physical data | |
| Formula | C10F20 |
| Molar mass | 500.078 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Perfluoro tert-butylcyclohexane is a perfluorinated chemical compound (or perfluorocarbon, PFC). It is a component of the empirical therapeutic oxygen carrier called Oxycyte.[1]
Chemical properties
Perfluoro tert-butyl-cyclo-hexane is a saturated alicyclic perfluorocarbon with the molecular formula C10F20.[2][3] Fluorocarbons are known for their strong gas-dissolving properties which, when used with oxygen, serve a dual role of healing the tissue as well as imaging. It fits the imaging role promisingly due to its biocompatibility and half-life. A similar compound, perfluoro-butane, is already used for ultrasound imaging.[4][5] Healing decompression sickness with oxygen has a similar mechanism of action to healing brain anemia, and so Oxycyte can be used for this.[6][7]
Oxycyte
Oxycyte is a perfluorocarbon (PFC) product composed of submicron particles of perfluoro(t-butylcyclohexane) in a 60% w/v concentration with an egg phospholipid emulsifier.[1]
Oxycyte was invented by Leland Clark and developed by Tenex Therapeutics, formerly Oxygen Biotherapeutics, Inc. and Synthetic Blood International.[8] It is designed to enhance oxygen delivery to damaged tissues. Through a collaborative agreement, Oxycyte (under the development code name of ABL-101) was being developed by Aurum Biosciences Ltd, with an initial indication in acute ischemic stroke.[9]
Tenex claims that Oxycyte can carry oxygen with up to 5 times the efficiency of hemoglobin when used as an intravenous emulsion, making it an effective means of transporting oxygen to tissues and carrying carbon dioxide to the lungs for disposal.[10] However, because Oxycyte is a PFC and not based on hemoglobin, it does not have the safety issues associated with hemoglobin-based products. There have been no adverse events in company clinical trials related to Oxycyte. Tenex believed Oxycyte has a very favorable risk-benefit profile for its potential indications.
Aurum Biosciences promoted Oxycyte as having potential use in multiple indications, including cardiology, oncology, and treating epilepsy and neurodegenerative diseases. These claims raised Tenex's stock price to its peak. Since then, the stock price has plummeted with no signs of recovery.[11][12]
Around September 2004, Oxycyte completed Phase I trials with few mild side effects.[13] Clinical interest in Oxycyte began to grow during this period, culminating in 2013.[14]
Aurum Biosciences received a Wellcome Trust HICF funding to conduct a Phase I clinical trial with Oxycyte in stroke patients. This study investigated both therapeutic potential and its ability to enhance the diagnostic potential of an MRI in suspected stroke patients.[15][16][17]
In September 2014, Oxygen Biotherapeutics announced the discontinuation of a Phase IIB trial for its Oxycyte drug candidate, citing "difficulties enrolling patients".[18] Clinical interest in Oxycyte has since tapered off.[14]
Oxycyte has been linked to potentially dangerous variations in blood viscosity.[19]
References
- 1 2 Haque A, Scultetus AH, Arnaud F, Dickson LJ, Chun S, McNamee G, et al. (December 2016). "The Emulsified PFC Oxycyte® Improved Oxygen Content and Lung Injury Score in a Swine Model of Oleic Acid Lung Injury (OALI)". Lung. 194 (6): 945–957. doi:10.1007/s00408-016-9941-9. PMID 27704259.
- ↑ Shaw R, Richard T (2006). "Chapter 27 – Rational Development of Oxyfluor". In Winslow RM (ed.). Blood Substitutes. Academic Press. p. 303. ISBN 978-0127597607.
- ↑ US 2013096190, Huvard G, Kiral R, Quitaro M, Thompson DP, Grossman A, Clauson G, Sandhu G, "Perfluorocarbon gel formulations", published 18 April 2013, assigned to Oxygen Biotherapeutics, Inc..
- ↑ Darçot E, Colotti R, Brennan D, Deuchar GA, Santosh C, van Heeswijk RB (January 2020). "A characterization of ABL-101 as a potential tracer for clinical fluorine-19 MRI". NMR in Biomedicine. 33 (1): e4212. doi:10.1002/nbm.4212. PMID 31724252. S2CID 208018877.
- ↑ "Ultrasound Contrast Agent Sonazoid® for Injection Released for Sale" (PDF). Daiichi Sankyo Company, Ltd. January 10, 2007.
- ↑ Mahon RT, Cronin WA, Bodo M, Tirumala S, Regis DP, Auker CR (January 2015). "Cardiovascular parameters in a mixed-sex swine study of severe decompression sickness treated with the emulsified perfluorocarbon Oxycyte". Journal of Applied Physiology. 118 (1): 71–79. doi:10.1152/japplphysiol.00727.2014. PMID 25342702.
- ↑ Mahon RT, Auker CR, Bradley SG, Mendelson A, Hall AA (March 2013). "The emulsified perfluorocarbon Oxycyte improves spinal cord injury in a swine model of decompression sickness". Spinal Cord. 51 (3): 188–192. doi:10.1038/sc.2012.135. PMID 23165506. S2CID 11694901.
- ↑ "Oxycyte | Tenax Therapeutics". www.tenaxthera.com. Retrieved 2017-01-24.
- ↑ "Aurum Biosciences". www.aurumbiosciences.com. Retrieved 2017-01-24.
- ↑ "Oxygen Biotherapeutics, Inc". Archived from the original on 2 April 2010.
- ↑ "Man Made Life Saver 50x Better Than Blood". December 15, 2006.
- ↑ "Tenax Therapeutics Stock Price Today (NASDAQ: TENX) Quote, Market Cap, Chart | WallStreetZen". www.wallstreetzen.com. Retrieved 2022-09-04.
- ↑ "Synthetic Blood International Announces Preliminary Analysis of Oxycyte Phase I Study Results - O-STA". o-sta.si. Retrieved 2022-09-04.
- 1 2 "Oxycyte - Search Results". PubMed. U.S. National Library of Medicine. Retrieved 2022-09-04.
- ↑ "Health Innovation Challenge Fund: projects we've funded". Wellcome. UK. Archived from the original on 2021-01-24. Retrieved 2017-01-24.
- ↑ "Technology and Products". Aurum Biosciences. Retrieved 2017-01-24.
- ↑ US 9144392, Santosh C, Brennan D, "Method of imaging metabolic function", issued 9 September 2015, assigned to The Greater Glasgow Health Board.
- ↑ "Oxygen Biotherapeutics Announces Halt of Oxycyte Phase IIb Traumatic Brain Injury Trial". businesswire.com. September 11, 2014.
- ↑ Arnaud F, Sanders K, Sieckmann D, Moon-Massat P (May 2016). "In vitro alteration of hematological parameters and blood viscosity by the perfluorocarbon: Oxycyte". International Journal of Hematology. 103 (5): 584–591. doi:10.1007/s12185-016-1955-9. PMID 26886450. S2CID 19287551.