Pafuramidine
 | |
| Names | |
|---|---|
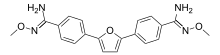
| IUPAC name
N′-Methoxy-4-[5-[4-[(Z)-N′-methoxycarbamimidoyl]phenyl]furan-2-yl]
benzenecarboximidamide | |
| Other names
DB289 | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C20H20N4O3 |
| Molar mass | 364.405 g·mol−1 |
| Pharmacology | |
| Oral | |
| Legal status |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pafuramidine (formulated as the maleic acid salt pafuramidine maleate) is an experimental drug for the treatment of pneumocystis pneumonia (PCP). In 2006, pafuramidine was given orphan drug status by the US Food and Drug Administration for PCP in patients with HIV/AIDS.[1] Preliminary clinical trials indicated that pafuramide was effective against pneumocystis pneumonia and had the potential for fewer side effects than the standard treatment with trimethoprim/sulfamethoxazole (TMP-SMX).[2]
Pafuramidine also reached Phase III clinical trials for the treatment of first stage African sleeping sickness, but development was halted in 2008 over concerns about kidney toxicity.[3][4]
References
- ↑ "US FDA Grants Immtech's Oral Drug Candidate Pafuramidine (DB289) Orphan Drug Status for Treatment of PCP". Drugs.com. November 21, 2006.
- ↑ Chen D, Marsh R, Aberg JA (2007). "Pafuramidine for Pneumocystis jiroveci pneumonia in HIV-infected individuals". Expert Review of Anti-infective Therapy. 5 (6): 921–8. doi:10.1586/14787210.5.6.921. PMID 18039076.
- ↑ "Pafuramidine maleate (DB289)". Swiss Tropical and Public Health Initiative.
- ↑ Harrill AH, Desmet KD, Wolf KK, Bridges AS, Eaddy JS, Kurtz CL, Hall JE, Paine MF, Tidwell RR, Watkins PB (2012). "A mouse diversity panel approach reveals the potential for clinical kidney injury due to DB289 not predicted by classical rodent models". Toxicol. Sci. 130 (2): 416–26. doi:10.1093/toxsci/kfs238. PMC 3498743. PMID 22940726.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.