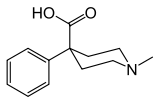
Pethidinic acid
 | |
| Clinical data | |
|---|---|
| Routes of administration | N/A |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H17NO2 |
| Molar mass | 219.284 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Pethidinic acid (meperidinic acid, pethidine intermediate C) is a 4-phenylpiperidine derivative that is both a metabolite of and a precursor to pethidine (meperidine).[1][2] It is scheduled by UN Single Convention on Narcotic Drugs. It is a Schedule II Narcotic controlled substance in the United States and has an ACSCN of 9234. The 2014 annual manufacturing quota was 6 grams. [3]
Pethidinic acid is a controlled drug because of its potential uses in manufacturing both pethidine itself and some of its substituted derivatives, but it has little opioid activity in its own right. Metabolism of pethidine to pethidinic acid is carried out mainly by the carboxylesterase enzyme hCE-1 in the liver,[4] and since the activity of this enzyme can vary between individuals, the rate and extent of pethidinic acid production can vary.[5][6]
Frank Wätjen used pethidinic acid as a precursor chemical to a heterocyclic moiety.[7]
See also
- Moramide intermediate
- Methadone intermediate
- Pethidine intermediate A
- Pethidine intermediate B (norpethidine)
References
- ↑ Tompsett SL (1962). "The detection and determination of pethidinic acid in urine". Acta Pharmacologica et Toxicologica. 19: 368–70. PMID 13985467.
- ↑ Chan K, Lau OW, Wong YC (April 1991). "Determination of pethidine and its major metabolites in human urine by gas chromatography". Journal of Chromatography. 565 (1–2): 247–54. doi:10.1016/0378-4347(91)80387-R. PMID 1874871.
- ↑ "Conversion Factors for Controlled Substances". DEA Diversion Control Division. Drug Enforcement Agency, U.S. Department of Justice.
- ↑ Zhang J, Burnell JC, Dumaual N, Bosron WF (July 1999). "Binding and hydrolysis of meperidine by human liver carboxylesterase hCE-1". The Journal of Pharmacology and Experimental Therapeutics. 290 (1): 314–8. PMID 10381793.
- ↑ Wainer IW, Stambaugh JE (January 1978). "GLC determination of meperidinic and normeperidinic acids in urine". Journal of Pharmaceutical Sciences. 67 (1): 116–8. doi:10.1002/jps.2600670131. PMID 619098.
- ↑ Odar-Cederlöf I, Boréus LO, Bondesson U, Holmberg L, Heyner L (1985). "Comparison of renal excretion of pethidine (meperidine) and its metabolites in old and young patients". European Journal of Clinical Pharmacology. 28 (2): 171–5. doi:10.1007/BF00609687. PMID 3987796. S2CID 20800555.
- ↑ EP 0285032, Wätjen, Frank, "4-Phenylpiperidine compounds and their preparation and use", published 1988-10-05, assigned to Ferrosan A.S.