Phosphatidylserine
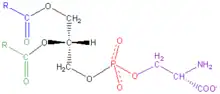 Components of phosphatidylserines: Blue, green: variable fatty acid groups Black: glycerol Red: phosphate Purple: serine | |
| Identifiers | |
|---|---|
| ChEBI | |
| ChemSpider |
|
| DrugBank | |
| KEGG | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phosphatidylserine (abbreviated Ptd-L-Ser or PS) is a phospholipid and is a component of the cell membrane.[1] It plays a key role in cell cycle signaling, specifically in relation to apoptosis. It is a key pathway for viruses to enter cells via apoptotic mimicry.[2] Its exposure on the outer surface of a membrane marks the cell for destruction via apoptosis.
Structure
Phosphatidylserine is a phospholipid—more specifically a glycerophospholipid—which consists of two fatty acids attached in ester linkage to the first and second carbon of glycerol and serine attached through a phosphodiester linkage to the third carbon of the glycerol.[3]
Phosphatidylserine sourced from plants differs in fatty acid composition from that sourced from animals.[4] It is commonly found in the inner (cytoplasmic) leaflet of biological membranes.[5] It is almost entirely found in the inner monolayer of the membrane with only less than 10% of it in the outer monolayer.
Introduction
Phosphatidylserine (PS) is the major acidic phospholipid class that accounts for 13–15 % of the phospholipids in the human cerebral cortex.[6] In the plasma membrane, PS is localized exclusively in the cytoplasmic leaflet where it forms part of protein docking sites necessary for the activation of several key signaling pathways. These include the Akt, protein kinase C (PKC) and Raf-1 signaling that is known to stimulate neuronal survival, neurite growth, and synaptogenesis.[7][8][9][10][11][12] Modulation of the PS level in the plasma membrane of neurons has a significant impact on these signaling processes.
Biosynthesis
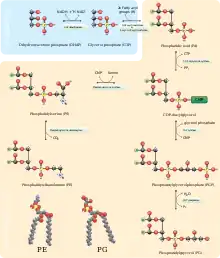
Phosphatidylserine is formed in bacteria (such as E. coli) through a displacement of cytidine monophosphate (CMP) through a nucleophilic attack by the hydroxyl functional group of serine. CMP is formed from CDP-diacylglycerol by PS synthase. Phosphatidylserine can eventually become phosphatidylethanolamine by the enzyme PS decarboxylase (forming carbon dioxide as a byproduct).[5] Similar to bacteria, yeast can form phosphatidylserine in an identical pathway.
In mammals, phosphatidylserine is instead derived from phosphatidylethanolamine or phosphatidylcholine through one of two Ca2+-dependent head-group exchange reactions in the endoplasmic reticulum. Both reactions require a serine but product an ethanolamine or choline, respectively. These are promoted by phosphatidylserine synthase 1 (PSS1) or 2 (PSS2).[5] Conversely, phosphatidylserine can also give rise to phosphatidylethanolamine and phosphatidylcholine, although in animals the pathway to generate phosphatidylcholine from phosphatidylserine only operates in the liver.[13]
Dietary sources
The average daily phosphatidylserine intake in a Western diet is estimated to be 130 mg.[14] Phosphatidylserine may be found in meat and fish. Only small amounts are found in dairy products and vegetables, with the exception of white beans and soy lecithin. Phosphatidylserine is found in soy lecithin at about 3% of total phospholipids.[15]
Table 1. Phosphatidylserine content in different foods.[16]
| Food | Content in mg/100 g |
|---|---|
| Bovine brain | 713 |
| Atlantic mackerel | 480 |
| Chicken heart | 414 |
| Atlantic herring | 360 |
| Eel | 335 |
| Offal (average value) | 305 |
| Pig's spleen | 239 |
| Pig's kidney | 218 |
| Tuna | 194 |
| Chicken leg, with skin, without bone | 134 |
| Chicken liver | 123 |
| White beans | 107 |
| Soft-shell clam | 87 |
| Chicken breast, with skin | 85 |
| Mullet | 76 |
| Veal | 72 |
| Beef | 69 |
| Pork | 57 |
| Pig's liver | 50 |
| Turkey leg, without skin or bone | 50 |
| Turkey breast without skin | 45 |
| Crayfish | 40 |
| Cuttlefish | 31 |
| Atlantic cod | 28 |
| Anchovy | 25 |
| Whole grain barley | 20 |
| European hake | 17 |
| European pilchard (sardine) | 16 |
| Trout | 14 |
| Rice (unpolished) | 3 |
| Carrot | 2 |
| Ewe's Milk | 2 |
| Cow's Milk (whole, 3.5% fat) | 1 |
| Potato | 1 |
Supplementation
Health claims
A panel of the European Food Safety Authority concluded that a cause and effect relationship cannot be established between the consumption of phosphatidylserine and “memory and cognitive functioning in the elderly”, “mental health/cognitive function” and “stress reduction and enhanced memory function”.[4] This conclusion follows because bovine brain cortex- and soy-based phosphatidylserine are different substances and might, therefore, have different biological activities. Therefore, the results of studies using phosphatidylserine from different sources cannot be generalized.[4]
Cognition
In May, 2003 the Food and Drug Administration gave "qualified health claim" status to phosphatidylserine thus allowing labels to state "consumption of phosphatidylserine may reduce the risk of dementia and cognitive dysfunction in the elderly" along with the disclaimer "very limited and preliminary scientific research suggests that phosphatidylserine may reduce the risk of cognitive dysfunction in the elderly."[17][18] According to the FDA, there is a lack of scientific agreement amongst qualified experts that a relationship exists between phosphatidylserine and cognitive function.[17]
More recent reviews have suggested that the relationship may be more robust,[19][20] though the mechanism remains unclear.[21] A 2020 meta-analysis of relevant clinical trials found that phosphatidylserine is likely effective for enhancing cognitive function in older people with mild cognitive impairment.[22] Some studies have suggested that whether the phosphatidylserine is plant- or animal-derived may have significance, with the FDA's statement applying specifically to soy-derived products.[17][23][24][25][26]
Safety
Initially, phosphatidylserine supplements were derived from bovine cortex. However, due to the risk of potential transfer of infectious diseases such as bovine spongiform encephalopathy (or "mad cow disease"), soy-derived supplements became an alternative.[23] A 2002 safety report determined supplementation in elderly people at a dosage of 200 mg three times daily to be safe.[27] Concerns about the safety of soy products persist, and some manufacturers of phosphatidylserine use sunflower lecithin instead of soy lecithin as a source of raw material production.
References
- ↑ Kannan, Muthukumar; Riekhof, Wayne R.; Voelker, Dennis R. (2015). "Transport of Phosphatidylserine from the Endoplasmic Reticulum to the Site of Phosphatidylserine Decarboxylase2 in Yeast". Traffic. 16 (2): 123–134. doi:10.1111/tra.12236. ISSN 1600-0854. PMID 25355612.
- ↑ Meertens L, Carnec X, Lecoin MP, Ramdasi R, Guivel-Benhassine F, Lew E, Lemke G, Schwartz O, Amara A (October 2012). "The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry". Cell Host & Microbe. 12 (4): 544–57. doi:10.1016/j.chom.2012.08.009. PMC 3572209. PMID 23084921.
- ↑ Nelson D, Cox M (2008). Lehninger Principles of biochemistry (5 ed.). W.H Freeman and company. pp. 350. ISBN 9781429208925.
- 1 2 3 EFSA Panel on Dietetic Products, Nutrition and Allergies (2010-10-01). "Scientific Opinion on the substantiation of health claims related to phosphatidyl serine (ID 552, 711, 734, 1632, 1927) pursuant to Article 13(1) of Regulation (EC) No 1924/2006". EFSA Journal. 8 (10): 1749. doi:10.2903/j.efsa.2010.1749. ISSN 1831-4732.
- 1 2 3 Verfasser., Nelson, David L. 1942- (16 March 2021). Lehninger principles of biochemistry. ISBN 978-1-319-38149-3. OCLC 1249676451.
{{cite book}}:|last=has generic name (help) - ↑ Svennerholm, Lars (September 1968). "Distribution and fatty acid composition of phosphoglycerides in normal human brain" (PDF). Journal of Lipid Research. 9 (5): 570–579. doi:10.1016/S0022-2275(20)42702-6. PMID 4302302. Retrieved 2021-06-14.
- ↑ Akbar, Mohammed; Calderon, Frances; Wen, Zhiming; Kim, Hee-Yong (2005-08-02). "Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival". Proceedings of the National Academy of Sciences of the United States of America. 102 (31): 10858–10863. doi:10.1073/pnas.0502903102. PMC 1182431. PMID 16040805.
- ↑ Huang, Bill X.; Akbar, Mohammed; Kevala, Karl; Kim, Hee-Yong (2011-03-21). "Phosphatidylserine is a critical modulator for Akt activation". Journal of Cell Biology. 192 (6): 979–992. doi:10.1083/jcb.201005100. PMC 3063130. PMID 21402788. Retrieved 2021-06-13.
- ↑ Kim, Hee-Yong; Akbar, Mohammed; Lau, Audrey; Edsall, Lisa (2000-11-10). "Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3): Role of phosphatidylserine in antiapoptotic effect". Journal of Biological Chemistry. 275 (45): 35215–35223. doi:10.1074/jbc.M004446200. PMID 10903316. Retrieved 2021-06-14.
- ↑ Kim, Hee-Yong (2007-06-29). "Novel metabolism of docosahexaenoic acid in neural cells". Journal of Biological Chemistry. 282 (26): 18661–18665. doi:10.1074/jbc.R700015200. PMID 17488715. Retrieved 2021-06-14.
- ↑ Kim, Hee-Yong; Akbar, Mohammed; Kim, Yang-Suk (2010). "Phosphatidylserine-dependent neuroprotective signaling promoted by docosahexaenoic acid". Prostaglandins, Leukotrienes and Essential Fatty Acids. 82 (4–6): 165–172. doi:10.1016/j.plefa.2010.02.025. PMC 3383770. PMID 20207120. Retrieved 2021-06-14.
- ↑ Newton, Alexandra C.; Keranen, Lisa M. (1994-05-31). "Phosphatidyl-L-serine is necessary for protein kinase C's high-affinity interaction with diacylglycerol-containing membranes". Biochemistry. 33 (21): 6651–6658. doi:10.1021/bi00187a035. PMID 8204602. Retrieved 2021-06-14.
- ↑ Christie WW (12 June 2014). "Phosphatidylcholine and Related Lipids: Structure, Occurrence, Biochemistry and Analysis" (PDF). The American Oil Chemists’ Society Lipid Library. Retrieved 20 April 2017.
- ↑ Souci SW, Fachmann E, Kraut H: Food Composition and Nutrition Tables. Stuttgart. 2000, Medpharm Scientific Publishers
- ↑ Miranda, Dalva T. S. Z.; Batista, Vanessa G.; Grando, Fernanda C. C.; Paula, Fernanda M.; Felício, Caroline A.; Rubbo, Gabriella F. S.; Fernandes, Luiz C.; Curi, Rui; Nishiyama, Anita (Dec 2008). "Soy lecithin supplementation alters macrophage phagocytosis and lymphocyte response to concanavalin A: a study in alloxan-induced diabetic rats". Cell Biochemistry and Function. 26 (8): 859–865. doi:10.1002/cbf.1517. ISSN 1099-0844. PMID 18846580. S2CID 9083077.
- ↑ Souci SW, Fachmann E, Kraut H (2008). Food Composition and Nutrition Tables. Medpharm Scientific Publishers Stuttgart.
- 1 2 3 Taylor CL (May 13, 2003). "Phosphatidylserine and Cognitive Dysfunction and Dementia (Qualified Health Claim: Final Decision Letter)". Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration. Retrieved 23 August 2014.
- ↑ "Summary of Qualified Health Claims Subject to Enforcement Discretion - Qualified Claims About Cognitive Function".
- ↑ Glade MJ, Smith K (June 2015). "Phosphatidylserine and the human brain". Nutrition. 31 (6): 781–6. doi:10.1016/j.nut.2014.10.014. PMID 25933483.
- ↑ Poddar, Jit; Pradhan, Munmun; Ganguly, Gargi; Chakrabarti, Sasanka (2019). "Biochemical deficits and cognitive decline in brain aging: Intervention by dietary supplements". Journal of Chemical Neuroanatomy. 95: 70–80. doi:10.1016/j.jchemneu.2018.04.002. ISSN 0891-0618. PMID 29678666. S2CID 5014367.
- ↑ Kim HY, Huang BX, Spector AA (October 2014). "Phosphatidylserine in the brain: metabolism and function". Progress in Lipid Research. 56: 1–18. doi:10.1016/j.plipres.2014.06.002. PMC 4258547. PMID 24992464.
- ↑ Tardner, P. (2020-08-28). "The effects of phosphatidylserine supplementation on memory function in older people: A review of clinical literature • International Journal of Environmental Science & Technology". International Journal of Environmental Science & Technology. Retrieved 2020-09-01.
- 1 2 Smith, Glenn (2 June 2014). "Can phosphatidylserine improve memory and cognitive function in people with Alzheimer's disease?". Mayo Clinic. Retrieved 23 August 2014.
- ↑ Crook TH, Klatz RM, eds. (1998). Treatment of Age-Related Cognitive Decline: Effects of Phosphatidylserine in Anti-Aging Medical Therapeutics. Vol. 2. Chicago: Health Quest Publications. pp. 20–29.
- ↑ Jorissen BL, Brouns F, Van Boxtel MP, Ponds RW, Verhey FR, Jolles J, Riedel WJ (2001). "The influence of soy-derived phosphatidylserine on cognition in age-associated memory impairment". Nutritional Neuroscience. 4 (2): 121–34. doi:10.1080/1028415X.2001.11747356. PMID 11842880. S2CID 9426593. Archived from the original on 2014-08-26. Retrieved 2014-08-23.
- ↑ Kato-Kataoka A, Sakai M, Ebina R, Nonaka C, Asano T, Miyamori T (November 2010). "Soybean-derived phosphatidylserine improves memory function of the elderly Japanese subjects with memory complaints". Journal of Clinical Biochemistry and Nutrition. 47 (3): 246–55. doi:10.3164/jcbn.10-62. PMC 2966935. PMID 21103034.
- ↑ Jorissen BL, Brouns F, Van Boxtel MP, Riedel WJ (October 2002). "Safety of soy-derived phosphatidylserine in elderly people". Nutritional Neuroscience. 5 (5): 337–43. doi:10.1080/1028415021000033802. PMID 12385596. S2CID 5688203.
External links
- DrugBank info page
- Phosphatidylserines at the US National Library of Medicine Medical Subject Headings (MeSH)