Radioresistance
Radioresistance is the level of ionizing radiation that organisms are able to withstand.
Ionizing-radiation-resistant organisms (IRRO) were defined as organisms for which the dose of acute ionizing radiation (IR) required to achieve 90% reduction (D10) is greater than 1000 gray (Gy) [1]
Radioresistance is surprisingly high in many organisms, in contrast to previously held views. For example, the study of environment, animals and plants around the Chernobyl disaster area has revealed an unexpected survival of many species, despite the high radiation levels. A Brazilian study in a hill in the state of Minas Gerais which has high natural radiation levels from uranium deposits, has also shown many radioresistant insects, worms and plants.[2][3] Certain extremophiles, such as the bacteria Deinococcus radiodurans and the tardigrades, can withstand large doses of ionizing radiation on the order of 5,000 Gy.[4][5][6]
Induced radioresistance
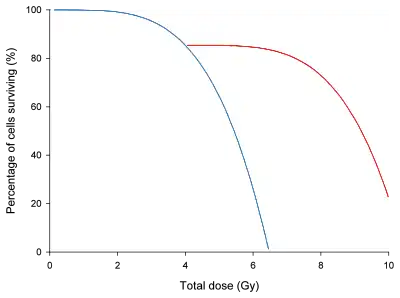
In the graph on left, a dose/survival curve for a hypothetical group of cells has been drawn with and without a rest time for the cells to recover. Other than the recovery time partway through the irradiation, the cells would have been treated identically.
Radioresistance may be induced by exposure to small doses of ionizing radiation. Several studies have documented this effect in yeast, bacteria, protozoa, algae, plants, insects, as well as in in vitro mammalian and human cells and in animal models. Several cellular radioprotection mechanisms may be involved, such as alterations in the levels of some cytoplasmic and nuclear proteins and increased gene expression, DNA repair and other processes. Also biophysical models presented general basics for this phenomenon.[7]
Many organisms have been found to possess a self-repair mechanism that can be activated by exposure to radiation in some cases. Two examples of this self-repair process in humans are described below.
Devair Alves Ferreira received a large dose (7.0 Gy) during the Goiânia accident, and lived, whereas his wife, who got a dose of 5.7 Gy, died. The most likely explanation is that his dose was fractionated into many smaller doses which were absorbed over a length of time while his wife stayed in the house more and was subjected to continuous irradiation without a break so giving the self repair mechanisms in her body less time to repair some of the damage done by the radiation. This resulted in her death. He also eventually died in 1994. In the same way some of the persons who worked in the basement of the wrecked Chernobyl have built up doses of 10 Gy, these workers received these doses in small fractions so the acute effects were avoided.
It has been found in radiation biology experiments that if a group of cells are irradiated then as the dose increases the number of cells which survive decrease. It has also been found that if a population of cells are given a dose before being set aside (without being irradiated) for a length of time before being irradiated again then the radiation has less of an ability to cause cell death. The human body contains many types of cells and a human can be killed by the loss of a single tissue in a vital organ. For many short term radiation deaths (3 days to 30 days) the loss of cells forming blood cells (bone marrow) and the cells in the digestive system (wall of the intestines) cause death.
Inheritance of radioresistance
There is strong evidence that radioresistance can be genetically determined and inherited, at least in some organisms. Heinrich Nöthel, a geneticist from the Freie Universität Berlin carried out the most extensive study about radioresistance mutations using the common fruit fly, Drosophila melanogaster, in a series of 14 publications.
Evolution of radioresistance
From the perspective of evolutionary history and causation, radioresistance does not appear to be an adaptive trait because there is no documented naturally occurring selection pressure that could have bestowed a fitness advantage to the ability for organisms to withstand doses of ionizing radiation in the range that several extremophile species have been observed to be capable of surviving.[8] This is primarily because the Earth's magnetic field shields all its inhabitants from solar cosmic radiation and galactic cosmic rays,[9] which are the two primary sources of ionizing radiation across our solar system,[10] and even including all documented terrestrial sources of ionizing radiation such as radon gas and primordial radionuclides at geographical locations considered to be natural high-level radiation sites, the yearly dose of natural background radiation[11] remains tens of thousands of times smaller than the levels of ionizing radiation that many highly radioresistant organisms can withstand.
One possible explanation for the existence of radioresistance is that it is an example of co-opted adaptation or exaptation, where radioresistance could be an indirect consequence of the evolution of a different, linked adaptation that has been positively selected for by evolution. For example, the desiccation-adaptation hypothesis proposes that the extreme temperatures present in the habitats of hyperthermophiles like Deinococcus radiodurans cause cellular damage that is virtually identical to damage typically caused by ionizing radiation, and that the cellular repair mechanisms that have evolved to repair this heat or desiccation damage are generalizable to radiation damage as well, allowing D. radiodurans to survive extreme doses of ionizing radiation.[12] Exposure to gamma radiation leads to cellular DNA damage including alterations in nitrogenous base-pairing, sugar-phosphate backbone damage, and double-stranded DNA lesions.[13] The extraordinarily efficient cellular repair mechanisms that Deinoccocus species like D. radiodurans have evolved to repair heat-damage are likely also capable of reversing the effects of DNA damage wrought by ionizing radiation, such as by piecing back together any components of their genome that have been fragmented by the radiation.[14][15][16]
Bacillus sp. producing unusually radiation (and peroxide) resistant spores, have been isolated from spacecraft assembly facilities, and are thought of as candidates that could ride piggyback on spacecraft through interplanetary transfer.[17][18][19][20][21] Genome analysis of some of these radiation resistant spore producers have thrown some light on the genetic traits that could be responsible for the resistances observed.[22] [23][24][25]
Radioresistance in radiation oncology
Radioresistance is also a term sometimes used in medicine (oncology) for cancer cells which are difficult to treat with radiotherapy. Radioresistance of cancer cells may be intrinsic or induced by the radiation therapy itself.
Radioresistance comparison
The comparison in the table below is only meant to give approximate indications of radioresistance for different species and should be taken with great caution. There are generally big differences in radioresistance for one species among experiments, due to the way radiation affects living tissues and to different experimental conditions. We should for example consider that because radiation impedes cell division, immature organisms are less resistant to radiations than adults, and adults are sterilized at doses much lower than that necessary to kill them. For example, for the insect parasitoid Habrobracon hebetor, the LD50 for haploid embryo during cleavage (1–3 hours of age) is 200 R, but about 4 hours later it is of 7,000 R (for X-ray intensity of 110 R/minute), and haploid (= male) embryos are more resistant than diploid (= female) embryos.[26] The mortality of adults H. hebetor exposed to a dose of 180,250 R is the same to this of a non-irradiated control group (food was not provided to either groups) (for 6,000 R/minute).[27][28] However, a lower dose of 102,000 R (for 6,000 R/minute) is sufficient to induce a state of lethargy in H. hebetor that is manifested by a complete cessation of activity, including cessation of feeding, and these individuals eventually let themselves starve to death.[28] And an even lower dose of 4,858 R (for 2,650 R/minute) is sufficient to sterilize adult female H. hebetor (sterility arises 3 days post-exposure).[29] Other important factors that influence the level of radioresistance include: The length of time during which a dose of radiation is delivered—with doses delivered during longer periods, or at time intervals, being associated with greatly reduced negative effects;[29][30] The feeding state of individuals—with pre-fed and post-fed individuals being more resistant to radiations compared to starved individuals;[29][30] The type of radiation used (e.g., tardigrades Milnesium tardigradum irradiated with heavy ions have a higher survival than when irradiated with gamma rays, for a same irradiation dose);[31] The physiological state of individuals (e.g., the tardigrade species Richtersius coronifer and Milnesium tardigradum are more resistant to gamma-ray radiation when in the hydrated state, and Macrobiotus areolatus is more resistant to X-ray radiation when in the anhydrobiotic state).[31] The way lethality is measured is also source of variation for the estimated radioresistance of a species. Irradiated specimens are not instantly killed, unless exposed to a very high dose (acute dose).[32] Therefore, irradiated specimens die over a certain period of time and lower irradiation doses correspond to longer survival. This means that the radiation dose LD50 fluctuates with the time at which it is measured. For example, the β radiation dose that causes 50% mortality in the American cockroach at 25 days post-exposure is 5,700 R, but to reach 50% mortality at 3 days post-exposure, 45,610 R are needed.[30] 25 days can represent a long survival period for short lived species, such as insects, but would represent a very short survival time for long lived species, such as mammals, so comparing survival of different species after the same amount of time post-exposure also poses some challenges of interpretation. These examples illustrate the many issues associated with comparison of radioresistance among species and the need for caution when doing so.
| Organism | Lethal dose | LD50 | LD100 | Class/Kingdom |
|---|---|---|---|---|
| Dog | 3.5 (LD50/30 days)[33] | Mammals | ||
| Human | 4–10[34] | 4.5[35] | 10[36] | Mammals |
| Rat | 7.5 | Mammals | ||
| Mouse | 4.5–12 | 8.6–9 | Mammals | |
| Rabbit | 8 (LD50/30 days)[33] | Mammals | ||
| Tortoise | 15 (LD50/30 days)[33] | Reptile | ||
| Goldfish | 20 (LD50/30 days)[33] | Fish | ||
| Escherichia coli | 60 | 60 | Bacteria | |
| German cockroach | 64[34] | Insects | ||
| Shellfish | 200 (LD50/30 days)[33] | - | ||
| Common fruit fly | 640[34] | Insects | ||
| C. elegans∗ | 160-200 [37] | ≫ 500-800[38][39] | Nematode | |
| Amoeba | 1,000 (LD50/30 days)[33] | - | ||
| Habrobracon hebetor | 1,800[27][28] | Insects | ||
| Milnesium tardigradum | 5,000[31] | Eutardigrade | ||
| Deinococcus radiodurans | 15,000[34] | Bacteria | ||
| Thermococcus gammatolerans | 30,000[34] | Archaea |
∗ While an LD50 has been reported for wild type C. elegans individuals, an upper lethal limit has not been established, rather "nearly all animals were alive with no indication of excess lethality up to 800 Gy, the highest dose... measured."[39]
See also
- Ex-Rad a radioprotective drug studied for its ability to protect against acute radiation syndrome
- CBLB502 a similar radioprotective drug, that protects against acute radiation syndrome, during radiotherapy.
- Radiosensitivity
- Background radiation
- Radiation hormesis
- Radiotrophic fungus
- Kojic acid
Notes and references
- ↑ Sghaier, Haïtham; Ghedira, Kaïs; Benkahla, Alia; Barkallah, Insaf (2008). "Basal DNA repair machinery is subject to positive selection in ionizing-radiation-resistant bacteria". BMC Genomics. 9: 297. doi:10.1186/1471-2164-9-297. PMC 2441631. PMID 18570673.
- ↑ Cordeiro, AR; Marques, EK; Veiga-Neto, AJ (1973). "Radioresistance of a natural population of Drosophila willistoni living in a radioactive environment". Mutation Research. 19 (3): 325–9. doi:10.1016/0027-5107(73)90233-9. PMID 4796403.
- ↑ Moustacchi, E (1965). "Induction by physical and chemical agents of mutations for radioresistance in Saccharomyces cerevisiae". Mutation Research. 2 (5): 403–12. doi:10.1016/0027-5107(65)90052-7. PMID 5878261.
- ↑ Moseley BEB; Mattingly A (1971). "Repair of irradiated transforming deoxyribonu- cleic acid in wild type and a radiation- sensitive mutant of Micrococcus radiodu- rans". J. Bacteriol. 105 (3): 976–83. doi:10.1128/JB.105.3.976-983.1971. PMC 248526. PMID 4929286.
- ↑ Murray RGE. 1992. The family Deino- coccaceae. In The Prokaryotes, ed. A Ballows, HG Truper, M Dworkin, W Harder, KH Schleifer 4:3732–44. New York: Springer-Verlag
- ↑ Ito H; Watanabe H; Takeshia M; Iizuka H (1983). "Isolation and identification of radiation-resistant cocci belonging to the genus Deinococcus from sewage sludges and animal feeds". Agricultural and Biological Chemistry. 47 (6): 1239–47. doi:10.1271/bbb1961.47.1239.
- ↑ Fornalski KW (2019). "Radiation adaptive response and cancer: from the statistical physics point of view". Physical Review E. 99 (2): 022139. Bibcode:2019PhRvE..99b2139F. doi:10.1103/PhysRevE.99.022139. PMID 30934317.
- ↑ Anitori, Roberto Paul (2012). Extremophiles: Microbiology and Biotechnology. Horizon Scientific Press. ISBN 9781904455981.
- ↑ Mukherjee, Saumitra (2008-12-03). "Cosmic Influence on the Sun-Earth Environment". Sensors (Basel, Switzerland). 8 (12): 7736–7752. Bibcode:2008Senso...8.7736M. doi:10.3390/s8127736. ISSN 1424-8220. PMC 3790986. PMID 27873955.
- ↑ Kennedy, Ann R. (2014-04-01). "Biological Effects of Space Radiation and Development of Effective Countermeasures". Life Sciences in Space Research. 1: 10–43. Bibcode:2014LSSR....1...10K. doi:10.1016/j.lssr.2014.02.004. ISSN 2214-5524. PMC 4170231. PMID 25258703.
- ↑ Shahbazi-Gahrouei, Daryoush; Gholami, Mehrdad; Setayandeh, Samaneh (2013-01-01). "A review on natural background radiation". Advanced Biomedical Research. 2 (1): 65. doi:10.4103/2277-9175.115821. ISSN 2277-9175. PMC 3814895. PMID 24223380.
- ↑ Mattimore, V.; Battista, J. R. (February 1996). "Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation". Journal of Bacteriology. 178 (3): 633–637. doi:10.1128/jb.178.3.633-637.1996. ISSN 0021-9193. PMC 177705. PMID 8550493.
- ↑ Friedberg, Errol C.; Friedberg, EC; Walker, GC; Walker, Graham C.; Siede, Wolfram; Wolfram, Siede (1995). DNA Repair and Mutagenesis. ASM Press. ISBN 9781555810887.
- ↑ Minton, K. W. (July 1994). "DNA repair in the extremely radioresistant bacterium Deinococcus radiodurans". Molecular Microbiology. 13 (1): 9–15. doi:10.1111/j.1365-2958.1994.tb00397.x. ISSN 0950-382X. PMID 7984097.
- ↑ Slade, Dea; Radman, Miroslav (March 2011). "Oxidative stress resistance in Deinococcus radiodurans". Microbiology and Molecular Biology Reviews. 75 (1): 133–191. doi:10.1128/MMBR.00015-10. ISSN 1098-5557. PMC 3063356. PMID 21372322.
- ↑ Agapov, A. A.; Kulbachinskiy, A. V. (October 2015). "Mechanisms of Stress Resistance and Gene Regulation in the Radioresistant Bacterium Deinococcus radiodurans". Biochemistry. Biokhimiia. 80 (10): 1201–1216. doi:10.1134/S0006297915100016. ISSN 1608-3040. PMID 26567564. S2CID 14981740.
- ↑ La Duc MT, Nicholson W, Kern R, Venkateswaran K (2003). "Microbial characterization of the Mars Odyssey spacecraft and its encapsulation facility". Environ Microbiol. 5 (10): 977–85. doi:10.1046/j.1462-2920.2003.00496.x. PMID 14510851.
- ↑ Link L, Sawyer J, Venkateswaran K, Nicholson W (February 2004). "Extreme spore UV resistance of Bacillus pumilus isolates obtained from an ultraclean Spacecraft Assembly Facility". Microb Ecol. 47 (2): 159–163. doi:10.1007/s00248-003-1029-4. PMID 14502417. S2CID 13416635.
- ↑ Kempf MJ, Chen F, Kern R, Venkateswaran K (June 2005). "Recurrent isolation of hydrogen peroxide- resistant spores of Bacillus pumilus from a spacecraft assembly facility". Astrobiology. 5 (3): 391–405. Bibcode:2005AsBio...5..391K. doi:10.1089/ast.2005.5.391. PMID 15941382.
- ↑ Newcombe DA, Schuerger AC, Benardini JN, Dickinson D, Tanner R, Venkateswaran K (December 2005). "Survival of spacecraft-associated microorganisms under simulated martian UV irradiation". Appl Environ Microbiol. 71 (12): 8147–8156. Bibcode:2005ApEnM..71.8147N. doi:10.1128/AEM.71.12.8147-8156.2005. PMC 1317311. PMID 16332797.
- ↑ Ghosh S, Osman S, Vaishampayan P, Venkateswaran K (2010). "Recurrent isolation of extremotolerant bacteria from the clean room where Phoenix spacecraft components were assembled" (PDF). Astrobiology. 10 (3): 325–35. Bibcode:2010AsBio..10..325G. doi:10.1089/ast.2009.0396. hdl:2027.42/85129. PMID 20446872.
- ↑ Gioia J, Yerrapragada S, Qin X, et al. (September 2007). "Paradoxical DNA Repair and Peroxide Resistance Gene Conservation in Bacillus pumilus SAFR-032". PLOS ONE. 2 (9:e928): e928. Bibcode:2007PLoSO...2..928G. doi:10.1371/journal.pone.0000928. PMC 1976550. PMID 17895969.
- ↑ Tirumalai MR, Rastogi R, Zamani N, O'Bryant Williams E, Allen S, Diouf F, Kwende S, Weinstock GM, Venkateswaran KJ, Fox GE (June 2013). "Candidate Genes That May Be Responsible for the Unusual Resistances Exhibited by Bacillus pumilus SAFR-032 Spores". PLOS ONE. 8 (6:e66012): e66012. Bibcode:2013PLoSO...866012T. doi:10.1371/journal.pone.0066012. PMC 3682946. PMID 23799069.
- ↑ Tirumalai MR, Fox GE (September 2013). "An ICEBs1-like element may be associated with the extreme radiation and desiccation resistance of Bacillus pumilus SAFR-032 spores". Extremophiles. 17 (5): 767–774. doi:10.1007/s00792-013-0559-z. PMID 23812891. S2CID 8675124.
- ↑ Tirumalai MR, Stepanov VG, Wünsche A, Montazari S, Gonzalez RO, Venkateswaran K, Fox GE (June 2018). "B. safensis FO-36bT and B. pumilus SAFR-032: A Whole Genome Comparison of two Spacecraft Assembly Facility Isolates". BMC Microbiol. 18 (57): 57. doi:10.1186/s12866-018-1191-y. PMC 5994023. PMID 29884123.
- ↑ Clark, AM; Mitchell, CJ (1952). "Effects of X-Rays upon Haploid and Diploid Embryos of Habrobracon". Biological Bulletin. 103 (2): 170–177. doi:10.2307/1538443. JSTOR 1538443.
- 1 2 Sullivan, R; Grosch, D (1953). "The radiation tolerance of an adult wasp". Nucleotics. 11: 21–23.
- 1 2 3 Grosch, DS (1954). "Induced lethargy and the radiation control of insects". Journal of Economic Entomology. 49 (5): 629–631. doi:10.1093/jee/49.5.629.
- 1 2 3 Grosch, DS; Sullivan, RL (1954). "The quantitative aspects of permanent and temporary sterility induced in female Habrobracon by X-Rays and β radiation". Radiation Research. 1 (3): 294–320. Bibcode:1954RadR....1..294G. doi:10.2307/3570374. JSTOR 3570374. PMID 13167339.
- 1 2 3 Wharton, DRA; Wharton, ML (1959). "The effect of radiation on the longevity of the cockroach, Periplaneta americana, as affected by dose, age, sex, and food intake". Radiation Research. 11 (4): 600–615. Bibcode:1959RadR...11..600W. doi:10.2307/3570814. JSTOR 3570814. PMID 13844254.
- 1 2 3 Horikawa DD; Sakashita T; Katagiri C; Watanabe M; et al. (2006). "Radiation tolerance in the tardigrade Milnesium tardigradum". International Journal of Radiation Biology. 82 (12): 843–8. doi:10.1080/09553000600972956. PMID 17178624. S2CID 25354328.
- ↑ Heidenthal, G (1945). "The occurrence of X-ray induced dominant lethal mutations in Habrobracon". Genetics. 30 (2): 197–205. doi:10.1093/genetics/30.2.197. PMC 1209282. PMID 17247153.
- 1 2 3 4 5 6 Radiochemistry and Nuclear Chemistry, G. Choppin, J-O. Liljenzin and J. Rydberg, edition three, page 481, ISBN 0-7506-7463-6
- 1 2 3 4 5 "Cockroaches & Radiation". 2006-02-23. Retrieved 2006-05-13.
- ↑ "Radiation Notes: Radiation Damage and Dose Measurement". Retrieved 2018-06-16.
- ↑ "CDC Radiation Emergencies, Acute Radiation Syndrome: A Fact Sheet for Physicians". Archived from the original on 2006-07-16.
- ↑ Hartman, P; Goldstein, P; Algarra, M; Hubbard, D; Mabery, J (1996). "The nematode Caenorhabditis elegans is up to 39 times more sensitive to gamma radiation generated from 137Cs than from 60Co". Mutat Res. 363 (3): 201–208. doi:10.1016/0921-8777(96)00012-2. PMID 8765161.
- ↑ Weidhaas, J.B.; Eisenmann, D.M.; Holub, J.M.; Nallur, S.V. (2006). "A Caenorhabditis elegans tissue model of radiation-induced reproductive cell death". Proc Natl Acad Sci USA. 103 (26): 9946–51. Bibcode:2006PNAS..103.9946W. doi:10.1073/pnas.0603791103. PMC 1502559. PMID 16788064.
- 1 2 Krisko, A.; Magali, L.; Radman, M.; Meselson, M. (2012). "Extreme anti-oxidant protection against ionizing radiation in bdelloid rotifers". Proc Natl Acad Sci USA. 109 (7): 2354–2357. Bibcode:2012PNAS..109.2354K. doi:10.1073/pnas.1119762109. PMC 3289372. PMID 22308443.
Further reading
- Joiner, M.C. (1994). "Induced Radioresistance: An Overview and Historical Perspective". International Journal of Radiation Biology. 65 (1): 79–84. doi:10.1080/09553009414550111. PMID 7905914.
- Clifton Ling, C.; Endlich, B. (1989). "Radioresistance Induced by Oncogenic Transformation". Radiation Research. 120 (2): 267–79. Bibcode:1989RadR..120..267L. doi:10.2307/3577713. JSTOR 3577713. PMID 2694214.
- Nothel, H. (1987). "Adaptation of Drosophila melanogaster Populations to High Mutation Pressure: Evolutionary Adjustment of Mutation Rates". Proceedings of the National Academy of Sciences. 84 (4): 1045–9. Bibcode:1987PNAS...84.1045N. doi:10.1073/pnas.84.4.1045. PMC 304358. PMID 3103121.
- Fornalski, K.W. (2016). "Radiation and evolution: from Lotka-Volterra equation to balance equation". International Journal of Low Radiation. 10 (3): 222–33. doi:10.1504/IJLR.2016.10002388.