Phenylacetic acid
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Phenylacetic acid | |
| Systematic IUPAC name
Phenylethanoic acid | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| 3DMet | |
Beilstein Reference |
1099647 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.002.862 |
| EC Number |
|
Gmelin Reference |
68976 |
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C8H8O2 |
| Molar mass | 136.15 g/mol |
| Density | 1.0809 g/cm3 |
| Melting point | 76 to 77 °C (169 to 171 °F; 349 to 350 K) |
| Boiling point | 265.5 °C (509.9 °F; 538.6 K) |
Solubility in water |
15 g/L |
| Acidity (pKa) | 4.31 (H2O)[1] |
Magnetic susceptibility (χ) |
-82.72·10−6 cm3/mol |
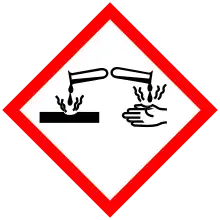
| Hazards | |
| GHS labelling: | |
Pictograms |
  |
Signal word |
Danger |
Hazard statements |
H318, H319 |
Precautionary statements |
P264, P280, P305+P351+P338, P310, P337+P313 |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related compounds |
Benzoic acid, Phenylpropanoic acid, Cinnamic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phenylacetic acid (PAA; conjugate base phenylacetate), also known by various synonyms, is an organic compound containing a phenyl functional group and a carboxylic acid functional group. It is a white solid with a strong honey-like odor. Endogenously, it is a catabolite of phenylalanine. As a commercial chemical, because it can be used in the illicit production of phenylacetone (used in the manufacture of substituted amphetamines), it is subject to controls in countries including the United States and China.[2]
Names
Synonyms include α-toluic acid, benzeneacetic acid, alpha tolylic acid, 2-phenylacetic acid, and β-phenylacetic acid.
Occurrence
Phenylacetic acid has been found to be an active auxin (a type of plant hormone),[3] found predominantly in fruits. However, its effect is much weaker than the effect of the basic auxin molecule indole-3-acetic acid. In addition the molecule is naturally produced by the metapleural gland of most ant species and used as an antimicrobial. It is also the oxidation product of phenethylamine in humans following metabolism by monoamine oxidase and subsequent metabolism of the intermediate product, phenylacetaldehyde, by the aldehyde dehydrogenase enzyme; these enzymes are also found in many other organisms.
Preparation
This compound may be prepared by the hydrolysis of benzyl cyanide:[4][5]

Applications
Phenylacetic acid is used in some perfumes, as it possesses a honey-like odor even in low concentrations. It is also used in penicillin G production and diclofenac production. It is also employed to treat type II hyperammonemia to help reduce the amounts of ammonia in a patient's bloodstream by forming phenylacetyl-CoA, which then reacts with nitrogen-rich glutamine to form phenylacetylglutamine. This compound is then excreted from the patient's body. It's also used in the illicit production of phenylacetone, which is used in the manufacture of methamphetamine.
The sodium salt of phenylacetic acid, sodium phenylacetate, is used as a pharmaceteutical drug for the treatment of urea cycle disorders, including as the combination drug sodium phenylacetate/sodium benzoate (Ammonul).[6]
Phenylacetic acid is used in the preparation of several pharmaceutical drugs, including camylofin, bendazol, triafungin, phenacemide, lorcainide, phenindione, and cyclopentolate.
In popular culture
In the crime drama Breaking Bad, phenylacetic acid is featured twice as a precursor to methamphetamine, first in the episode titled A No-Rough-Stuff-Type Deal, then in Salud.
See also
- Cathinone
- Methyl phenylacetate
References
- ↑ Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. pp. 5–89. ISBN 978-1498754286.
- ↑ "List of Regulated Drug Precursor Chemicals in China". Archived from the original on 17 August 2015. Retrieved 27 April 2015.
- ↑ Wightman, F.; Lighty, D. L. (1982). "Identification of phenylacetic acid as a natural auxin in the shoots of higher plants". Physiologia Plantarum. 55 (1): 17–24. doi:10.1111/j.1399-3054.1982.tb00278.x.
- ↑ Adams R.; Thal, A. F. (1922). "Phenylacetic acid". Organic Syntheses. 2: 59.; Collective Volume, vol. 1, p. 436
- ↑ Wenner, W. (1952). "Phenylacetamide". Organic Syntheses. 32: 92.; Collective Volume, vol. 4, p. 760
- ↑ "Sodium Phenylacetate and Sodium Benzoate Monograph for Professionals". Drugs.com. Retrieved 16 November 2019.

