Syncytiotrophoblast
| Syncytiotrophoblast | |
|---|---|
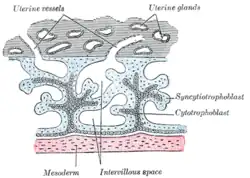 Primary chorionic villi. Diagrammatic. | |
 Secondary chorionic villi. Diagrammatic. | |
| Details | |
| Carnegie stage | 5a |
| Days | 8 |
| Identifiers | |
| Latin | syncitiotrophoblastus |
| TE | E6.0.1.1.4.0.2 |
| FMA | 83040 |
| Anatomical terminology | |
Syncytiotrophoblast (from the Greek 'syn'- "together"; 'cytio'- "of cells"; 'tropho'- "nutrition"; 'blast'- "bud") is the epithelial covering of the highly vascular embryonic placental villi, which invades the wall of the uterus to establish nutrient circulation between the embryo and the mother. It is a multi-nucleate, terminally differentiated syncytium, extending to 13 cm.
Function
It is the outer layer of the trophoblasts and actively invades the uterine wall, rupturing maternal capillaries and thus establishing an interface between maternal blood and embryonic extracellular fluid, facilitating passive exchange of material between the mother and the embryo.
The syncytial property is important since the mother's immune system includes white blood cells that are able to migrate into tissues by "squeezing" in between cells. If they were to reach the fetal side of the placenta many foreign proteins would be recognised, triggering an immune reaction. However the syncytium acts as a giant cell so there are no gaps for immune cells to migrate through.
One way in which it accomplishes this task is by suppressing the expression of immunity-related genes HLA-A and HLA-B, which are classically known to be expressed by all nucleated cells.[1] These genes normally express the MHC-I ligand that acts as a major binding mechanism for T-cells. By decreasing the translation of these gene products, the syncytiotrophoblast reduces the chances of an attack by the maternal immune system mediated by T-cells.[1]
The syncytiotrophoblast secretes progesterone and leptin in addition to human chorionic gonadotropin (hCG) and human placental lactogen (HPL); hCG prevents degeneration of the corpus luteum. Progesterone serves to maintain the integrity of the uterine lining and, until the syncytiotrophoblast is mature enough to secrete enough progesterone to support pregnancy (in the fourth month of embryonic development), it is aided by the corpus luteum graviditatis.[2]
Formation

The syncytiotrophoblast lacks proliferative capacity and instead is maintained by fusion of underlying cytotrophoblast cells. This fusion is assisted by syncytin, a protein that was integrated into mammalian genomes from an endogenous retrovirus.[3]
Additional images
 Section through embryonic area of Vespertilio murinus to show the formation of the amniotic cavity.
Section through embryonic area of Vespertilio murinus to show the formation of the amniotic cavity.
See also
- Cytotrophoblast
- Intermediate trophoblast
- Syncytium
References
- 1 2 Jay Iams; Creasy, Robert K.; Resnik, Robert; Robert Reznik (2004). Maternal-fetal medicine.
- ↑ Langman's Medical Embryology, 10th Edition. T.W. Sadler. p. 34
- ↑ Mi, S (Feb 17, 2000). "Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis". Nature. 403 (6771): 785–789. Bibcode:2000Natur.403..785M. doi:10.1038/35001608. PMID 10693809. S2CID 4367889.
Tony M. Plant, Anthony J. Zeleznik: "Knobil and Neill's Physiology of Reproduction: Two-Volume Set" p 1790
External links
- Histology image: 19908loa – Histology Learning System at Boston University - "Female Reproductive System: placental villi"
- Nature (Journal) Diagram
- Musicki B, Pepe G, Albrecht E (1997). "Functional differentiation of placental syncytiotrophoblasts during baboon pregnancy: developmental expression of chorionic somatomammotropin messenger ribonucleic acid and protein levels". J Clin Endocrinol Metab. 82 (12): 4105–10. doi:10.1210/jc.82.12.4105. PMID 9398722. Article
- Diagram at McGill