Adderall
 | |
 Top: racemic amphetamine skeleton Bottom: (D)-amphetamine ball-and-stick model | |
| Combination of | |
|---|---|
| amphetamine aspartate monohydrate | 25% – stimulant (12.5% levo; 12.5% dextro) |
| amphetamine sulfate | 25% – stimulant (12.5% levo; 12.5% dextro) |
| dextroamphetamine saccharate | 25% – stimulant (0% levo; 25% dextro) |
| dextroamphetamine sulfate | 25% – stimulant (0% levo; 25% dextro) |
| Names | |
| Trade names | Adderall, Adderall XR, Mydayis, others |
| Clinical data | |
| Dependence risk | Moderate[3] |
| Pregnancy category |
|
| Routes of use | Oral, insufflation, rectal, sublingual |
| External links | |
| AHFS/Drugs.com | Monograph |
| US NLM | Adderall |
| MedlinePlus | a601234 |
| Legal | |
| License data | |
| Legal status |
|
Adderall is a trade names[note 2] for a combination drug containing four salts of amphetamine. Other trade names for this combination also exist. The mixture is composed of equal parts racemic amphetamine and dextroamphetamine, which produces a (3:1) ratio between dextroamphetamine and levoamphetamine, the two enantiomers of amphetamine. Both enantiomers are stimulants, but differ enough to give Adderall an effects profile distinct from those of racemic amphetamine or dextroamphetamine,[1][2] which are marketed as Evekeo and Dexedrine/Zenzedi, respectively.[1][6][7] Adderall is used in the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy. It is also used as an athletic performance enhancer, cognitive enhancer, appetite suppressant, and recreationally as an aphrodisiac and euphoriant. It is a central nervous system (CNS) stimulant of the phenethylamine class.[1]
Adderall is generally well tolerated and effective in treating the symptoms of ADHD and narcolepsy. At therapeutic doses, Adderall causes emotional and cognitive effects such as euphoria, change in sex drive, increased wakefulness, and improved cognitive control. At these doses, it induces physical effects such as a faster reaction time, fatigue resistance, and increased muscle strength. In contrast, much larger doses of Adderall can impair cognitive control, cause rapid muscle breakdown, provoke panic attacks, or induce a psychosis (e.g., paranoia, delusions, hallucinations). The side effects of Adderall vary widely among individuals, but most commonly include insomnia, dry mouth, loss of appetite, and weight loss. The risk of developing an addiction or dependence is insignificant when Adderall is used as prescribed at fairly low daily doses, such as those used for treating ADHD; however, the routine use of Adderall in larger daily doses poses a significant risk of addiction or dependence due to the pronounced reinforcing effects that are present at high doses. Recreational doses of amphetamine are generally much larger than prescribed therapeutic doses, and carry a far greater risk of serious adverse effects.[sources 1]
The two amphetamine enantiomers that compose Adderall (levoamphetamine and dextroamphetamine) alleviate the symptoms of ADHD and narcolepsy by increasing the activity of the neurotransmitters norepinephrine and dopamine in the brain, which results in part from their interactions with human trace amine-associated receptor 1 (hTAAR1) and vesicular monoamine transporter 2 (VMAT2) in neurons. Dextroamphetamine is a more potent CNS stimulant than levoamphetamine, but levoamphetamine has slightly stronger cardiovascular and peripheral effects and a longer elimination half-life than dextroamphetamine. The levoamphetamine component of Adderall has been reported to improve the treatment response in some individuals relative to dextroamphetamine alone. Adderall's active ingredient, amphetamine, shares many chemical and pharmacological properties with the human trace amines, particularly phenethylamine and N-methylphenethylamine, the latter of which is a positional isomer of amphetamine.[sources 2] In 2018, Adderall was the 24th most commonly prescribed medication in the United States, with more than 25 million prescriptions.[27][28]
Uses

Medical
Adderall is used to treat attention deficit hyperactivity disorder (ADHD) and narcolepsy (a sleep disorder).[29][9] {{#section-h:Amphetamine|Medical}}
Available forms
Adderall is available as immediate-release (IR) tablets or two different extended-release (XR) formulations.[9][30] The extended-release capsules are generally used in the morning.[31] A shorter, 12-hour extended-release formulation is available under the brand Adderall XR and is designed to provide a therapeutic effect and plasma concentrations identical to taking two doses 4 hours apart.[30] The longer extended-release formulation, approved for 16 hours, is available under the brand Mydayis. In the United States, the immediate and extended release formulations of Adderall are both available as generic drugs,[32][33] while Mydayis is available only as a brand-name drug.
Performance
{{#section-h:Amphetamine|Enhancing performance}}
Adderall has been banned in the National Football League (NFL), Major League Baseball (MLB), National Basketball Association (NBA), and the National Collegiate Athletics Association (NCAA).[34] In leagues such as the NFL, there is a very rigorous process required to obtain an exemption to this rule even when the athlete has been medically prescribed the drug by their physician.[34]
Recreational
Adderall has high potential for misuse as a recreational drug.[35][36][37] Adderall tablets can either be swallowed, crushed and snorted, or dissolved in water and injected.[38] Injection into the bloodstream can be dangerous because insoluble fillers within the tablets can block small blood vessels.[38]
Many postsecondary students have reported using Adderall for study purposes in different parts of the developed world.[37] Among these students, some of the risk factors for misusing ADHD stimulants recreationally include: possessing deviant personality characteristics (i.e., exhibiting delinquent or deviant behavior), inadequate accommodation of special needs, basing one's self-worth on external validation, low self-efficacy, earning poor grades, and suffering from an untreated mental health disorder.[37]
Contraindications
Side effects
The side effects of Adderall are many and varied, but the amount of substance consumed is the primary factor in determining the likelihood and severity of side effects.[12][23] Adderall is currently approved for long-term therapeutic use by the USFDA.[12] Recreational use of Adderall generally involves far larger doses and is therefore significantly more dangerous, involving a much greater risk of serious adverse drug effects than dosages used for therapeutic purposes.[23] {{#section-h:Amphetamine|Adverse effects}}
Overdose
An amphetamine overdose can lead to many different symptoms, but is rarely fatal with appropriate care.[49][43][50] The severity of overdose symptoms increases with dosage and decreases with drug tolerance to amphetamine.[51][43] Tolerant individuals have been known to take as much as 5 grams of amphetamine in a day, which is roughly 100 times the maximum daily therapeutic dose.[43] Symptoms of a moderate and extremely large overdose are listed below; fatal amphetamine poisoning usually also involves convulsions and coma.[42][51] In 2013, overdose on amphetamine, methamphetamine, and other compounds implicated in an "amphetamine use disorder" resulted in an estimated 3,788 deaths worldwide (3,425–4,145 deaths, 95% confidence).[note 6][52]
| System | Minor or moderate overdose[42][51][43] | Severe overdose[sources 3] |
|---|---|---|
| Cardiovascular |
| |
| Central nervous system |
|
|
| Musculoskeletal |
| |
| Respiratory |
|
|
| Urinary |
|
|
| Other |
|
|
Interactions
- Monoamine oxidase inhibitors (MAOIs) taken with amphetamine may result in a hypertensive crisis if taken within two weeks after last use of an MAOI type drug.[55]
- Inhibitors of enzymes that directly metabolize amphetamine (particularly CYP2D6 and FMO3) will prolong the elimination of amphetamine and increase drug effects.[55][56][57]
- Serotonergic drugs (such as most antidepressants) co-administered with amphetamine increases the risk of serotonin syndrome.[57]
- Stimulants and antidepressants (sedatives and depressants) may increase (decrease) the drug effects of amphetamine, and vice versa.[55]
- Gastrointestinal and urinary pH affect the absorption and elimination of amphetamine, respectively. Gastrointestinal alkalizing (acidifying) agents increase the absorption of amphetamine. Urinary alkalizing (acidifying) agents increase concentration of non-ionized (ionized) species, decreasing (increasing) urinary excretion.[55]
- Proton-pump inhibitors (PPIs) modify the absorption of Adderall XR and Mydayis.[55][57]
- Zinc supplementation may reduce the minimum effective dose of amphetamine when it is used for the treatment of ADHD.[note 7][61]
Pharmacology
Pharmacodynamics of amphetamine in a dopamine neuron
|
Mechanism of action
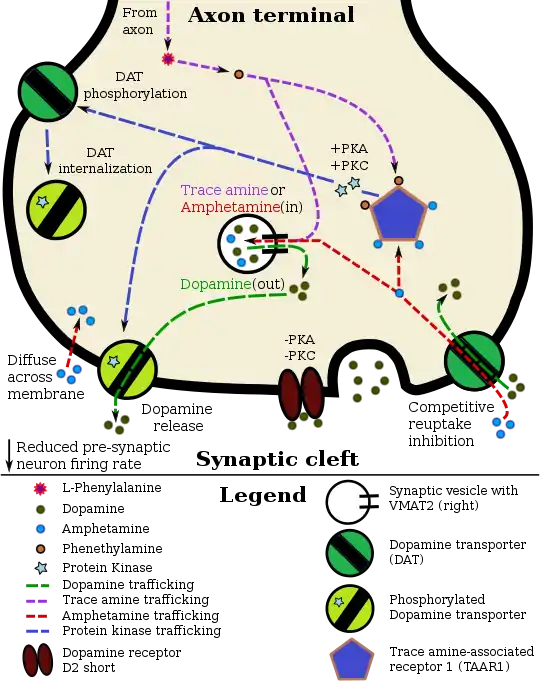
Amphetamine, the active ingredient of Adderall, works primarily by increasing the activity of the neurotransmitters dopamine and norepinephrine in the brain.[19][67] It also triggers the release of several other hormones (e.g., epinephrine) and neurotransmitters (e.g., serotonin and histamine) as well as the synthesis of certain neuropeptides (e.g., cocaine and amphetamine regulated transcript (CART) peptides).[21][68] Both active ingredients of Adderall, dextroamphetamine and levoamphetamine, bind to the same biological targets,[23][24] but their binding affinities (that is, potency) differ somewhat.[23][24] Dextroamphetamine and levoamphetamine are both potent full agonists (activating compounds) of trace amine-associated receptor 1 (TAAR1) and interact with vesicular monoamine transporter 2 (VMAT2), with dextroamphetamine being the more potent agonist of TAAR1.[24] Consequently, dextroamphetamine produces more CNS stimulation than levoamphetamine;[24][69] however, levoamphetamine has slightly greater cardiovascular and peripheral effects.[23] It has been reported that certain children have a better clinical response to levoamphetamine.[25][26]
In the absence of amphetamine, VMAT2 will normally move monoamines (e.g., dopamine, histamine, serotonin, norepinephrine, etc.) from the intracellular fluid of a monoamine neuron into its synaptic vesicles, which store neurotransmitters for later release (via exocytosis) into the synaptic cleft.[21] When amphetamine enters a neuron and interacts with VMAT2, the transporter reverses its direction of transport, thereby releasing stored monoamines inside synaptic vesicles back into the neuron's intracellular fluid.[21] Meanwhile, when amphetamine activates TAAR1, the receptor causes the neuron's cell membrane-bound monoamine transporters (i.e., the dopamine transporter, norepinephrine transporter, or serotonin transporter) to either stop transporting monoamines altogether (via transporter internalization) or transport monoamines out of the neuron;[20] in other words, the reversed membrane transporter will push dopamine, norepinephrine, and serotonin out of the neuron's intracellular fluid and into the synaptic cleft.[20] In summary, by interacting with both VMAT2 and TAAR1, amphetamine releases neurotransmitters from synaptic vesicles (the effect from VMAT2) into the intracellular fluid where they subsequently exit the neuron through the membrane-bound, reversed monoamine transporters (the effect from TAAR1).[20][21]
Pharmacokinetics
{{#section-h:Amphetamine|Pharmacokinetics}}
Pharmacomicrobiomics
The human metagenome (i.e., the genetic composition of an individual and all microorganisms that reside on or within the individual's body) varies considerably between individuals.[70][71] Since the total number of microbial and viral cells in the human body (over 100 trillion) greatly outnumbers human cells (tens of trillions),[note 8][70][72] there is considerable potential for interactions between drugs and an individual's microbiome, including: drugs altering the composition of the human microbiome, drug metabolism by microbial enzymes modifying the drug's pharmacokinetic profile, and microbial drug metabolism affecting a drug's clinical efficacy and toxicity profile.[70][71][73] The field that studies these interactions is known as pharmacomicrobiomics.[70]
Similar to most biomolecules and other orally administered xenobiotics (i.e., drugs), amphetamine is predicted to undergo promiscuous metabolism by human gastrointestinal microbiota (primarily bacteria) prior to absorption into the blood stream.[73] The first amphetamine-metabolizing microbial enzyme, tyramine oxidase from a strain of E. coli commonly found in the human gut, was identified in 2019.[73] This enzyme was found to metabolize amphetamine, tyramine, and phenethylamine with roughly the same binding affinity for all three compounds.[73]Related endogenous compounds
{{#section-h:Amphetamine|Related endogenous compounds}}
Society, and culture
Cost
In 2018, Adderall was the 24th most commonly prescribed medication in the United States, with more than 25 million prescription[27][28]
.svg.png.webp) Adderall costs (US)
Adderall costs (US).svg.png.webp) Adderall prescriptions (US)
Adderall prescriptions (US)
History
The pharmaceutical company Rexar reformulated their popular weight loss drug Obetrol following its mandatory withdrawal from the market in 1973 under the Kefauver Harris Amendment to the Federal Food, Drug, and Cosmetic Act due to the results of the Drug Efficacy Study Implementation (DESI) program (which indicated a lack of efficacy). The new formulation simply replaced the two methamphetamine components with dextroamphetamine and amphetamine components of the same weight (the other two original dextroamphetamine and amphetamine components were preserved), preserved the Obetrol branding, and despite it utterly lacking FDA approval, it still made it onto the market and was marketed and sold by Rexar for a number of years.
In 1994 Richwood Pharmaceuticals acquired Rexar and began promoting Obetrol as a treatment for ADHD (and later narcolepsy as well), now marketed under the new brand name of Adderall, a contraction of the phrase "A.D.D. for All" intended to convey that "it was meant to be kind of an inclusive thing" for marketing purposes.[74] The FDA cited the company for numerous significant CGMP violations related to Obetrol discovered during routine inspections following the acquisition (including issuing a formal warning letter for the violations), then later issued a second formal warning letter to Richwood Pharmaceuticals specifically due to violations of "the new drug and misbranding provisions of the FD&C Act". Following extended discussions with Richwood Pharmaceuticals regarding the resolution of a large number of issues related to the company's numerous violations of FDA regulations, the FDA formally approved the first Obetrol labeling/sNDA revisions in 1996, including a name change to Adderall and a restoration of its status as an approved drug product.[75][76] In 1997 Richwood Pharmaceuticals was acquired by Shire Pharmaceuticals in a $186 million transaction.[74]
Richwood Pharmaceuticals, which later merged with Shire plc, introduced the current Adderall brand in 1996 as an instant-release tablet.[77] In 2006, Shire agreed to sell rights to the Adderall name for the instant-release form of the medication to Duramed Pharmaceuticals.[78] DuraMed Pharmaceuticals was acquired by Teva Pharmaceuticals in 2008 during their acquisition of Barr Pharmaceuticals, including Barr's Duramed division.[79]
The first generic version of Adderall IR was introduced to market in 2002.[4] Later on, Barr and Shire reached a settlement agreement permitting Barr to offer a generic form of the extended-release drug beginning in April 2009.[4][80]
Commercial formulation
Chemically, Adderall is a mixture of four amphetamine salts; specifically, it is composed of equal parts (by mass) of amphetamine aspartate monohydrate, amphetamine sulfate, dextroamphetamine sulfate, and dextroamphetamine saccharate.[30] This drug mixture has slightly stronger CNS effects than racemic amphetamine due to the higher proportion of dextroamphetamine.[20][23] Adderall is produced as both an immediate release (IR) and extended release (XR) formulation.[4][9][30] As of December 2013, ten different companies produced generic Adderall IR, while Teva Pharmaceutical Industries, Actavis, and Barr Pharmaceuticals manufactured generic Adderall XR.[4] As of 2013, Shire plc, the company that held the original patent for Adderall and Adderall XR, still manufactured brand name Adderall XR, but not Adderall IR.[4]
Comparison to other formulations
Adderall is one of several formulations of pharmaceutical amphetamine, including singular or mixed enantiomers and as an enantiomer prodrug. The table below compares these medications (based on US approved forms):
| drug | formula | molecular mass [note 9] |
amphetamine base [note 10] |
amphetamine base in equal doses |
doses with equal base content [note 11] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (g/mol) | (percent) | (30 mg dose) | ||||||||
| total | base | total | dextro- | levo- | dextro- | levo- | ||||
| dextroamphetamine sulfate[82][83] | (C9H13N)2•H2SO4 | 368.49 |
270.41 |
73.38% |
73.38% |
— |
22.0 mg |
— |
30.0 mg | |
| amphetamine sulfate[84] | (C9H13N)2•H2SO4 | 368.49 |
270.41 |
73.38% |
36.69% |
36.69% |
11.0 mg |
11.0 mg |
30.0 mg | |
| Adderall | 62.57% |
47.49% |
15.08% |
14.2 mg |
4.5 mg |
35.2 mg | ||||
| 25% | dextroamphetamine sulfate[82][83] | (C9H13N)2•H2SO4 | 368.49 |
270.41 |
73.38% |
73.38% |
— |
|||
| 25% | amphetamine sulfate[84] | (C9H13N)2•H2SO4 | 368.49 |
270.41 |
73.38% |
36.69% |
36.69% |
|||
| 25% | dextroamphetamine saccharate[85] | (C9H13N)2•C6H10O8 | 480.55 |
270.41 |
56.27% |
56.27% |
— |
|||
| 25% | amphetamine aspartate monohydrate[86] | (C9H13N)•C4H7NO4•H2O | 286.32 |
135.21 |
47.22% |
23.61% |
23.61% |
|||
| lisdexamfetamine dimesylate[87] | C15H25N3O•(CH4O3S)2 | 455.49 |
135.21 |
29.68% |
29.68% |
— |
8.9 mg |
— |
74.2 mg | |
| amphetamine base suspension[88] | C9H13N | 135.21 |
135.21 |
100% |
76.19% |
23.81% |
22.9 mg |
7.1 mg |
22.0 mg | |
Legal status
- In Canada, amphetamines are in Schedule I of the Controlled Drugs and Substances Act, and can only be obtained by prescription.[89]
- In Japan, the use, production, and import of any medicine containing amphetamine are prohibited.[90]
- In South Korea, amphetamines are prohibited.[91]
- In Taiwan, amphetamines including Adderall are Schedule 2 drugs with a minimum five years prison term for possession.[92] Only Ritalin can be legally prescribed for treatment of ADHD.
- In Thailand, amphetamines are classified as Type 1 Narcotics.[93]
- In the United Kingdom, amphetamines are regarded as Class B drugs. The maximum penalty for unauthorized possession is five years in prison and an unlimited fine. The maximum penalty for illegal supply is 14 years in prison and an unlimited fine.[94]
- In the United States, amphetamine is a Schedule II prescription drug, classified as a CNS stimulant.[95]
- Internationally, amphetamine is in Schedule II of the Convention on Psychotropic Substances.[96][97]
See also
Notes
- ↑ Salts of racemic amphetamine and dextroamphetamine are mixed in a (1:1) ratio to produce this drug. Because the racemate is composed of equal parts dextroamphetamine and levoamphetamine, this drug can also be described as a mixture of the D and (L)-enantiomers of amphetamine in a (3:1) ratio, although none of the components of the mixture are levoamphetamine salts.[1][2]
- ↑ The trade name Adderall is used primarily throughout this article because the four-salt composition of the drug makes its nonproprietary name (dextroamphetamine sulfate 25%, dextroamphetamine saccharate 25%, amphetamine sulfate 25%, and amphetamine aspartate 25%) excessively lengthy.[4] Mydayis is a relatively new trade name that is not commonly used to refer generally to the mixture.[5]
- ↑ The statements supported by the USFDA come from prescribing information, which is the copyrighted intellectual property of the manufacturer and approved by the USFDA. USFDA contraindications are not necessarily intended to limit medical practice but limit claims by pharmaceutical companies.[39]
- ↑ According to one review, amphetamine can be prescribed to individuals with a history of abuse provided that appropriate medication controls are employed, such as requiring daily pick-ups of the medication from the prescribing physician.[40]
- ↑ In individuals who experience sub-normal height and weight gains, a rebound to normal levels is expected to occur if stimulant therapy is briefly interrupted.[46][47][48] The average reduction in final adult height from 3 years of continuous stimulant therapy is 2 cm.[48]
- ↑ The 95% confidence interval indicates that there is a 95% probability that the true number of deaths lies between 3,425 and 4,145.
- ↑ The human dopamine transporter contains a high affinity extracellular zinc binding site which, upon zinc binding, inhibits dopamine reuptake and amplifies amphetamine-induced dopamine efflux in vitro.[58][59][60] The human serotonin transporter and norepinephrine transporter do not contain zinc binding sites.[60]
- ↑ There is substantial variation in microbiome composition and microbial concentrations by anatomical site.[70][71] Fluid from the human colon – which contains the highest concentration of microbes of any anatomical site – contains approximately one trillion (10^12) bacterial cells/ml.[70]
- ↑ For uniformity, molecular masses were calculated using the Lenntech Molecular Weight Calculator[81] and were within 0.01g/mol of published pharmaceutical values.
- ↑ Amphetamine base percentage = molecular massbase / molecular masstotal. Amphetamine base percentage for Adderall = sum of component percentages / 4.
- ↑ dose = (1 / amphetamine base percentage) × scaling factor = (molecular masstotal / molecular massbase) × scaling factor. The values in this column were scaled to a 30 mg dose of dextroamphetamine sulfate. Due to pharmacological differences between these medications (e.g., differences in the release, absorption, conversion, concentration, differing effects of enantiomers, half-life, etc.), the listed values should not be considered equipotent doses.
- Image legend
Reference notes
References
- 1 2 3 4 Heal, David J; Smith, Sharon L; Gosden, Jane; Nutt, David J (28 March 2013). "Amphetamine, past and present – a pharmacological and clinical perspective". Journal of Psychopharmacology. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
- 1 2 Joyce, B. Matthew; Glaser, Paul E. A.; Gerhardt, Greg A. (10 October 2006). "Adderall® produces increased striatal dopamine release and a prolonged time course compared to amphetamine isomers". Psychopharmacology. 191 (3): 669–677. doi:10.1007/s00213-006-0550-9. PMID 17031708. S2CID 20283057.
- ↑
- 1 2 3 4 5 6 "National Drug Code Amphetamine Search Results". National Drug Code Directory. United States Food and Drug Administration. Archived from the original on 16 December 2013. Retrieved 16 December 2013.
- ↑
- ↑ "Pharmacology". Evekeo CII (amphetamine sulfate) HCP. Arbor Pharmaceuticals, LLC. Archived from the original on 21 September 2020. Retrieved 2 May 2020.
- ↑ "Prescribing Information & Medication Guide" (PDF). Zenzedi® (dextroamphetamine sulfate, USP). Arbor Pharmaceuticals LLC. Archived (PDF) from the original on 11 November 2020. Retrieved 2 May 2020.
- ↑
- 1 2 3 4 "Adderall- dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablet". DailyMed. Teva Pharmaceuticals USA, Inc. 8 November 2019. Archived from the original on 2 October 2019. Retrieved 22 December 2019.
- ↑
- ↑
- 1 2 3
- ↑
- ↑
- ↑
- ↑
- ↑ Stolerman IP (2010). Stolerman IP (ed.). Encyclopedia of Psychopharmacology. Berlin, Germany; London, England: Springer. p. 78. ISBN 9783540686989.
- ↑ Howell, L (2008). "Monoamine transporters and psychostimulant addiction". Biochemical Pharmacology. 75 (1): 196–217. doi:10.1016/j.bcp.2007.08.003. PMID 17825265.
- 1 2 Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. pp. 154–157. ISBN 9780071481274.
- 1 2 3 4 5 6 7 8 9 10 Miller GM (January 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". J. Neurochem. 116 (2): 164–76. doi:10.1111/j.1471-4159.2010.07109.x. PMC 3005101. PMID 21073468.
- 1 2 3 4 5 6 7 Eiden LE, Weihe E (January 2011). "VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse". Ann. N. Y. Acad. Sci. 1216 (1): 86–98. Bibcode:2011NYASA1216...86E. doi:10.1111/j.1749-6632.2010.05906.x. PMC 4183197. PMID 21272013.
VMAT2 is the CNS vesicular transporter for not only the biogenic amines DA, NE, EPI, 5-HT, and HIS, but likely also for the trace amines TYR, PEA, and thyronamine (THYR) ... [Trace aminergic] neurons in mammalian CNS would be identifiable as neurons expressing VMAT2 for storage, and the biosynthetic enzyme aromatic amino acid decarboxylase (AADC).
- ↑ Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- 1 2 3 4 5 6 7
- 1 2 3 4 5 Lewin AH, Miller GM, Gilmour B (December 2011). "Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class". Bioorg. Med. Chem. 19 (23): 7044–7048. doi:10.1016/j.bmc.2011.10.007. PMC 3236098. PMID 22037049.
- 1 2 Anthony, E. (11 November 2013). Explorations in Child Psychiatry. Springer Science & Business Media. pp. 93–94. ISBN 9781468421279. Archived from the original on 21 May 2016. Retrieved 28 April 2015.
- 1 2 Arnold LE (2000). "Methyiphenidate vs. Amphetamine: Comparative review". Journal of Attention Disorders. 3 (4): 200–211. doi:10.1177/108705470000300403. S2CID 15901046.
- 1 2 "The Top 300 Drugs of 2021". ClinCalc. Archived from the original on 18 March 2020. Retrieved 18 February 2021.
- 1 2 "Dextroamphetamine; Dextroamphetamine Saccharate; Amphetamine; Amphetamine Aspartate - Drug Usage Statistics". ClinCalc. Archived from the original on 1 March 2021. Retrieved 18 February 2021.
- ↑ Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present – a pharmacological and clinical perspective". Journal of Psychopharmacology. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
The intravenous use of d-amphetamine and other stimulants still pose major safety risks to the individuals indulging in this practice. Some of this intravenous abuse is derived from the diversion of ampoules of d-amphetamine, which are still occasionally prescribed in the UK for the control of severe narcolepsy and other disorders of excessive sedation. ... For these reasons, observations of dependence and abuse of prescription d-amphetamine are rare in clinical practice, and this stimulant can even be prescribed to people with a history of drug abuse provided certain controls, such as daily pick-ups of prescriptions, are put in place (Jasinski and Krishnan, 2009b).
- 1 2 3 4 "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. Archived (PDF) from the original on 30 December 2013. Retrieved 30 December 2013.
- ↑ Truven Health Analytics. "Amphetamine/Dextroamphetamine (By mouth)". PubMed Health. Micromedex Consumer Medication Information. Archived from the original on 11 October 2014. Retrieved 4 September 2015.
- ↑ "Generic Adderall Availability". Drugs.com. Archived from the original on 28 May 2020. Retrieved 6 February 2020.
- ↑ "Generic Adderall XR Availability". Drugs.com. Archived from the original on 6 February 2020. Retrieved 6 February 2020.
- 1 2 Leon Moore, David. "Do pro sports leagues have an Adderall problem?". USA TODAY. Archived from the original on 23 November 2014. Retrieved 4 May 2014.
- ↑ "Commonly Abused Prescription Drugs Chart". National Institute on Drug Abuse. Archived from the original on 1 May 2012. Retrieved 7 May 2012.
- ↑ "Stimulant ADHD Medications – Methylphenidate and Amphetamines". National Institute on Drug Abuse. Archived from the original on 2 May 2012. Retrieved 7 May 2012.
- 1 2 3 Abelman, Dor David (6 October 2017). "Mitigating risks of students use of study drugs through understanding motivations for use and applying harm reduction theory: a literature review". Harm Reduction Journal. 14 (1): 68. doi:10.1186/s12954-017-0194-6. ISSN 1477-7517. PMC 5639593. PMID 28985738.
- 1 2 "National Institute on Drug Abuse. 2009. Stimulant ADHD Medications – Methylphenidate and Amphetamines". National Institute on Drug Abuse. Archived from the original on 12 March 2013. Retrieved 27 February 2013.
- ↑ Kessler S (January 1996). "Drug therapy in attention-deficit hyperactivity disorder". Southern Medical Journal. 89 (1): 33–38. doi:10.1097/00007611-199601000-00005. PMID 8545689.
statements on package inserts are not intended to limit medical practice. Rather they are intended to limit claims by pharmaceutical companies. ... the FDA asserts explicitly, and the courts have upheld that clinical decisions are to be made by physicians and patients in individual situations.
- ↑ Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present – a pharmacological and clinical perspective". Journal of Psychopharmacology. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
The intravenous use of d-amphetamine and other stimulants still pose major safety risks to the individuals indulging in this practice. Some of this intravenous abuse is derived from the diversion of ampoules of d-amphetamine, which are still occasionally prescribed in the UK for the control of severe narcolepsy and other disorders of excessive sedation. ... For these reasons, observations of dependence and abuse of prescription d-amphetamine are rare in clinical practice, and this stimulant can even be prescribed to people with a history of drug abuse provided certain controls, such as daily pick-ups of prescriptions, are put in place (Jasinski and Krishnan, 2009b).
- 1 2 3 "Evekeo- amphetamine sulfate tablet". DailyMed. Arbor Pharmaceuticals, LLC. 14 August 2019. Archived from the original on 10 July 2018. Retrieved 22 December 2019.
- 1 2 3 4 5 6 7 8 9 "Adderall XR- dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine sulfate and amphetamine aspartate capsule, extended release". DailyMed. Shire US Inc. 17 July 2019. Retrieved 22 December 2019.
- 1 2 3 4 5 6 7 8 9 10 Heedes G, Ailakis J. "Amphetamine (PIM 934)". INCHEM. International Programme on Chemical Safety. Archived from the original on 18 April 2019. Retrieved 24 June 2014.
- ↑ Feinberg SS (November 2004). "Combining stimulants with monoamine oxidase inhibitors: a review of uses and one possible additional indication". The Journal of Clinical Psychiatry. 65 (11): 1520–1524. doi:10.4088/jcp.v65n1113. PMID 15554766.
- ↑ Stewart JW, Deliyannides DA, McGrath PJ (June 2014). "How treatable is refractory depression?". Journal of Affective Disorders. 167: 148–152. doi:10.1016/j.jad.2014.05.047. PMID 24972362.
- ↑ Huang YS, Tsai MH (July 2011). "Long-term outcomes with medications for attention-deficit hyperactivity disorder: current status of knowledge". CNS Drugs. 25 (7): 539–554. doi:10.2165/11589380-000000000-00000. PMID 21699268.
Several other studies,[97-101] including a meta-analytic review[98] and a retrospective study,[97] suggested that stimulant therapy in childhood is associated with a reduced risk of subsequent substance use, cigarette smoking and alcohol use disorders. ... Recent studies have demonstrated that stimulants, along with the non-stimulants atomoxetine and extended-release guanfacine, are continuously effective for more than 2-year treatment periods with few and tolerable adverse effects. The effectiveness of long-term therapy includes not only the core symptoms of ADHD, but also improved quality of life and academic achievements. The most concerning short-term adverse effects of stimulants, such as elevated blood pressure and heart rate, waned in long-term follow-up studies. ... The current data do not support the potential impact of stimulants on the worsening or development of tics or substance abuse into adulthood. In the longest follow-up study (of more than 10 years), lifetime stimulant treatment for ADHD was effective and protective against the development of adverse psychiatric disorders.
- ↑ Millichap JG (2010). "Chapter 9: Medications for ADHD". In Millichap JG (ed.). Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD (2nd ed.). New York, USA: Springer. p. 112. ISBN 9781441913968.
Table 9.2 Dextroamphetamine formulations of stimulant medication
Dexedrine [Peak:2–3 h] [Duration:5–6 h] ...
Adderall [Peak:2–3 h] [Duration:5–7 h]
Dexedrine spansules [Peak:7–8 h] [Duration:12 h] ...
Adderall XR [Peak:7–8 h] [Duration:12 h]
Vyvanse [Peak:3–4 h] [Duration:12 h] - 1 2 Vitiello B (April 2008). "Understanding the risk of using medications for attention deficit hyperactivity disorder with respect to physical growth and cardiovascular function". Child and Adolescent Psychiatric Clinics of North America. 17 (2): 459–474. doi:10.1016/j.chc.2007.11.010. PMC 2408826. PMID 18295156.
- ↑ Stahl SM (March 2017). "Amphetamine (D,L)". Prescriber's Guide: Stahl's Essential Psychopharmacology (6th ed.). Cambridge, United Kingdom: Cambridge University Press. pp. 45–51. ISBN 9781108228749. Archived from the original on 8 June 2019. Retrieved 5 August 2017.
- 1 2 Spiller HA, Hays HL, Aleguas A (June 2013). "Overdose of drugs for attention-deficit hyperactivity disorder: clinical presentation, mechanisms of toxicity, and management". CNS Drugs. 27 (7): 531–543. doi:10.1007/s40263-013-0084-8. PMID 23757186.
Amphetamine, dextroamphetamine, and methylphenidate act as substrates for the cellular monoamine transporter, especially the dopamine transporter (DAT) and less so the norepinephrine (NET) and serotonin transporter. The mechanism of toxicity is primarily related to excessive extracellular dopamine, norepinephrine, and serotonin.
- 1 2 3 4 Westfall DP, Westfall TC (2010). "Miscellaneous Sympathomimetic Agonists". In Brunton LL, Chabner BA, Knollmann BC (eds.). Goodman & Gilman's Pharmacological Basis of Therapeutics (12th ed.). New York, USA: McGraw-Hill. ISBN 9780071624428.
- ↑ Collaborators (2015). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013" (PDF). The Lancet. 385 (9963): 117–171. doi:10.1016/S0140-6736(14)61682-2. PMC 4340604. PMID 25530442. Retrieved 3 March 2015.
Amphetamine use disorders ... 3,788 (3,425–4,145)
- ↑ Greene SL, Kerr F, Braitberg G (October 2008). "Review article: amphetamines and related drugs of abuse". Emergency Medicine Australasia. 20 (5): 391–402. doi:10.1111/j.1742-6723.2008.01114.x. PMID 18973636.
- ↑ Albertson TE (2011). "Amphetamines". In Olson KR, Anderson IB, Benowitz NL, Blanc PD, Kearney TE, Kim-Katz SY, Wu AH (eds.). Poisoning & Drug Overdose (6th ed.). New York: McGraw-Hill Medical. pp. 77–79. ISBN 9780071668330.
- 1 2 3 4 5 "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. December 2013. pp. 8–10. Archived (PDF) from the original on 30 December 2013. Retrieved 30 December 2013.
- ↑
- 1 2 3 "Mydayis Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. June 2017. pp. 1–21. Archived (PDF) from the original on 9 June 2019. Retrieved 8 August 2017.
- ↑ Krause J (April 2008). "SPECT and PET of the dopamine transporter in attention-deficit/hyperactivity disorder". Expert Rev. Neurother. 8 (4): 611–625. doi:10.1586/14737175.8.4.611. PMID 18416663. S2CID 24589993.
Zinc binds at ... extracellular sites of the DAT [103], serving as a DAT inhibitor. In this context, controlled double-blind studies in children are of interest, which showed positive effects of zinc [supplementation] on symptoms of ADHD [105,106]. It should be stated that at this time [supplementation] with zinc is not integrated in any ADHD treatment algorithm.
- ↑ Sulzer D (February 2011). "How addictive drugs disrupt presynaptic dopamine neurotransmission". Neuron. 69 (4): 628–649. doi:10.1016/j.neuron.2011.02.010. PMC 3065181. PMID 21338876.
They did not confirm the predicted straightforward relationship between uptake and release, but rather that some compounds including AMPH were better releasers than substrates for uptake. Zinc, moreover, stimulates efflux of intracellular [3H]DA despite its concomitant inhibition of uptake (Scholze et al., 2002).
- 1 2 Scholze P, Nørregaard L, Singer EA, Freissmuth M, Gether U, Sitte HH (June 2002). "The role of zinc ions in reverse transport mediated by monoamine transporters". J. Biol. Chem. 277 (24): 21505–21513. doi:10.1074/jbc.M112265200. PMID 11940571.
The human dopamine transporter (hDAT) contains an endogenous high affinity Zn2+ binding site with three coordinating residues on its extracellular face (His193, His375, and Glu396). ... Although Zn2+ inhibited uptake, Zn2+ facilitated [3H]MPP+ release induced by amphetamine, MPP+, or K+-induced depolarization specifically at hDAT but not at the human serotonin and the norepinephrine transporter (hNET). ... Surprisingly, this amphetamine-elicited efflux was markedly enhanced, rather than inhibited, by the addition of 10 μM Zn2+ to the superfusion buffer (Fig. 2 A, open squares). We stress that Zn2+ per se did not affect basal efflux (Fig. 2 A). ... In many brain regions, Zn2+ is stored in synaptic vesicles and co-released together with glutamate; under basal conditions, the extracellular levels of Zn2+ are low (∼10 nM; see Refs. 39, 40). Upon neuronal stimulation, however, Zn2+ is co-released with the neurotransmitters and, consequently, the free Zn2+ concentration may transiently reach values that range from 10–20 μM (10) up to 300 μM (11). The concentrations of Zn2+ shown in this study, required for the stimulation of dopamine release (as well as inhibition of uptake), covered this physiologically relevant range, with maximum stimulation occurring at 3–30 μM. It is therefore conceivable that the action of Zn2+ on hDAT does not merely reflect a biochemical peculiarity but that it is physiologically relevant. ... Thus, when Zn2+ is co-released with glutamate, it may greatly augment the efflux of dopamine.
- ↑ Scassellati C, Bonvicini C, Faraone SV, Gennarelli M (October 2012). "Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses". J. Am. Acad. Child Adolesc. Psychiatry. 51 (10): 1003–1019.e20. doi:10.1016/j.jaac.2012.08.015. PMID 23021477.
Although we did not find a sufficient number of studies suitable for a meta-analysis of PEA and ADHD, three studies20,57,58 confirmed that urinary levels of PEA were significantly lower in patients with ADHD compared with controls. ... Administration of D-amphetamine and methylphenidate resulted in a markedly increased urinary excretion of PEA,20,60 suggesting that ADHD treatments normalize PEA levels. ... Similarly, urinary biogenic trace amine PEA levels could be a biomarker for the diagnosis of ADHD,20,57,58 for treatment efficacy,20,60 and associated with symptoms of inattentivenesss.59 ... With regard to zinc supplementation, a placebo controlled trial reported that doses up to 30 mg/day of zinc were safe for at least 8 weeks, but the clinical effect was equivocal except for the finding of a 37% reduction in amphetamine optimal dose with 30 mg per day of zinc.110
- ↑ Sulzer D, Cragg SJ, Rice ME (August 2016). "Striatal dopamine neurotransmission: regulation of release and uptake". Basal Ganglia. 6 (3): 123–148. doi:10.1016/j.baga.2016.02.001. PMC 4850498. PMID 27141430.
Despite the challenges in determining synaptic vesicle pH, the proton gradient across the vesicle membrane is of fundamental importance for its function. Exposure of isolated catecholamine vesicles to protonophores collapses the pH gradient and rapidly redistributes transmitter from inside to outside the vesicle. ... Amphetamine and its derivatives like methamphetamine are weak base compounds that are the only widely used class of drugs known to elicit transmitter release by a non-exocytic mechanism. As substrates for both DAT and VMAT, amphetamines can be taken up to the cytosol and then sequestered in vesicles, where they act to collapse the vesicular pH gradient.
- ↑ Ledonne A, Berretta N, Davoli A, Rizzo GR, Bernardi G, Mercuri NB (July 2011). "Electrophysiological effects of trace amines on mesencephalic dopaminergic neurons". Front. Syst. Neurosci. 5: 56. doi:10.3389/fnsys.2011.00056. PMC 3131148. PMID 21772817.
Three important new aspects of TAs action have recently emerged: (a) inhibition of firing due to increased release of dopamine; (b) reduction of D2 and GABAB receptor-mediated inhibitory responses (excitatory effects due to disinhibition); and (c) a direct TA1 receptor-mediated activation of GIRK channels which produce cell membrane hyperpolarization.
- ↑ "TAAR1". GenAtlas. University of Paris. 28 January 2012. Retrieved 29 May 2014.
• tonically activates inwardly rectifying K(+) channels, which reduces the basal firing frequency of dopamine (DA) neurons of the ventral tegmental area (VTA)
- ↑ Underhill SM, Wheeler DS, Li M, Watts SD, Ingram SL, Amara SG (July 2014). "Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons". Neuron. 83 (2): 404–416. doi:10.1016/j.neuron.2014.05.043. PMC 4159050. PMID 25033183.
AMPH also increases intracellular calcium (Gnegy et al., 2004) that is associated with calmodulin/CamKII activation (Wei et al., 2007) and modulation and trafficking of the DAT (Fog et al., 2006; Sakrikar et al., 2012). ... For example, AMPH increases extracellular glutamate in various brain regions including the striatum, VTA and NAc (Del Arco et al., 1999; Kim et al., 1981; Mora and Porras, 1993; Xue et al., 1996), but it has not been established whether this change can be explained by increased synaptic release or by reduced clearance of glutamate. ... DHK-sensitive, EAAT2 uptake was not altered by AMPH (Figure 1A). The remaining glutamate transport in these midbrain cultures is likely mediated by EAAT3 and this component was significantly decreased by AMPH
- ↑ Vaughan RA, Foster JD (September 2013). "Mechanisms of dopamine transporter regulation in normal and disease states". Trends Pharmacol. Sci. 34 (9): 489–496. doi:10.1016/j.tips.2013.07.005. PMC 3831354. PMID 23968642.
AMPH and METH also stimulate DA efflux, which is thought to be a crucial element in their addictive properties [80], although the mechanisms do not appear to be identical for each drug [81]. These processes are PKCβ– and CaMK–dependent [72, 82], and PKCβ knock-out mice display decreased AMPH-induced efflux that correlates with reduced AMPH-induced locomotion [72].
- ↑
- ↑ "Amphetamine: Biomolecular Interactions and Pathways". PubChem Compound. National Center for Biotechnology Information. Archived from the original on 13 October 2013. Retrieved 13 October 2013.
- ↑ Smith RC, Davis JM (June 1977). "Comparative effects of d-amphetamine, l-amphetamine, and methylphenidate on mood in man". Psychopharmacology. 53 (1): 1–12. doi:10.1007/bf00426687. PMID 407607. S2CID 37967136.
- 1 2 3 4 5 6 ElRakaiby M, Dutilh BE, Rizkallah MR, Boleij A, Cole JN, Aziz RK (July 2014). "Pharmacomicrobiomics: the impact of human microbiome variations on systems pharmacology and personalized therapeutics". Omics. 18 (7): 402–414. doi:10.1089/omi.2014.0018. PMC 4086029. PMID 24785449.
The hundred trillion microbes and viruses residing in every human body, which outnumber human cells and contribute at least 100 times more genes than those encoded on the human genome (Ley et al., 2006), offer an immense accessory pool for inter-individual genetic variation that has been underestimated and largely unexplored (Savage, 1977; Medini et al., 2008; Minot et al., 2011; Wylie et al., 2012). ... Meanwhile, a wealth of literature has long been available about the biotransformation of xenobiotics, notably by gut bacteria (reviewed in Sousa et al., 2008; Rizkallah et al., 2010; Johnson et al., 2012; Haiser and Turnbaugh, 2013). This valuable information is predominantly about drug metabolism by unknown human-associated microbes; however, only a few cases of inter-individual microbiome variations have been documented [e.g., digoxin (Mathan et al., 1989) and acetaminophen (Clayton et al., 2009)].
- 1 2 3 Cho I, Blaser MJ (March 2012). "The human microbiome: at the interface of health and disease". Nature Reviews Genetics. 13 (4): 260–270. doi:10.1038/nrg3182. PMC 3418802. PMID 22411464.
The composition of the microbiome varies by anatomical site (Figure 1). The primary determinant of community composition is anatomical location: interpersonal variation is substantial23,24 and is higher than the temporal variability seen at most sites in a single individual25. ... How does the microbiome affect the pharmacology of medications? Can we "micro-type" people to improve pharmacokinetics and/or reduce toxicity? Can we manipulate the microbiome to improve pharmacokinetic stability?
- ↑ Hutter T, Gimbert C, Bouchard F, Lapointe FJ (2015). "Being human is a gut feeling". Microbiome. 3: 9. doi:10.1186/s40168-015-0076-7. PMC 4359430. PMID 25774294.
Some metagenomic studies have suggested that less than 10% of the cells that comprise our bodies are Homo sapiens cells. The remaining 90% are bacterial cells. The description of this so-called human microbiome is of great interest and importance for several reasons. For one, it helps us redefine what a biological individual is. We suggest that a human individual is now best described as a super-individual in which a large number of different species (including Homo sapiens) coexist.
- 1 2 3 4 Kumar K, Dhoke GV, Sharma AK, Jaiswal SK, Sharma VK (January 2019). "Mechanistic elucidation of amphetamine metabolism by tyramine oxidase from human gut microbiota using molecular dynamics simulations". Journal of Cellular Biochemistry. 120 (7): 11206–11215. doi:10.1002/jcb.28396. PMID 30701587.
Particularly in the case of the human gut, which harbors a large diversity of bacterial species, the differences in microbial composition can significantly alter the metabolic activity in the gut lumen.4 The differential metabolic activity due to the differences in gut microbial species has been recently linked with various metabolic disorders and diseases.5–12 In addition to the impact of gut microbial diversity or dysbiosis in various human diseases, there is an increasing amount of evidence which shows that the gut microbes can affect the bioavailability and efficacy of various orally administrated drug molecules through promiscuous enzymatic metabolism.13,14 ... The present study on the atomistic details of amphetamine binding and binding affinity to the tyramine oxidase along with the comparison with two natural substrates of this enzyme namely tyramine and phenylalanine provides strong evidence for the promiscuity‐based metabolism of amphetamine by the tyramine oxidase enzyme of E. coli. The obtained results will be crucial in designing a surrogate molecule for amphetamine that can help either in improving the efficacy and bioavailability of the amphetamine drug via competitive inhibition or in redesigning the drug for better pharmacological effects. This study will also have useful clinical implications in reducing the gut microbiota caused variation in the drug response among different populations.
- 1 2 Schwarz, Alan (14 December 2013). "The Selling of Attention Deficit Disorder". The New York Times. ISSN 0362-4331. Archived from the original on 1 March 2015. Retrieved 22 April 2017.
- ↑ "(Collection of internal FDA information pertaining to the topic of Obetrol/Adderall)" (PDF). www.accessdata.fda.gov. US Food and Drug Administration. Archived (PDF) from the original on 17 May 2017. Retrieved 27 April 2017.
- ↑ "REGULATORY NEWS: Richwood's Adderall". Health News Daily. 22 February 1996. Archived from the original on 23 May 2016. Retrieved 29 May 2013.
- ↑ "APPROVAL LETTER" (PDF). United States Food and Drug Administration. Archived (PDF) from the original on 22 August 2013. Retrieved 30 December 2013.
- ↑ "August 2006 News Archives: Barr and Shire Sign Three Agreements". GenericsWeb. Archived from the original on 8 February 2014. Retrieved 30 December 2013.
WOODCLIFF LAKE, N.J., Aug. 14 /PRNewswire-FirstCall/ – Barr Pharmaceuticals, Inc. today announced that its subsidiary Duramed Pharmaceuticals, Inc. and Shire plc have signed a Product Acquisition Agreement for ADDERALL(R) (immediate-release mixed amphetamine salts) tablets and a Product Development Agreement for six proprietary products, and that its subsidiary Barr Laboratories, Inc. (Barr) has signed a Settlement and License Agreement relating to the resolution of two pending patent cases involving Shire's ADDERALL XR(R) ...
- ↑ "Teva Completes Acquisition of Barr". Drugs.com. Archived from the original on 8 March 2012. Retrieved 31 October 2011.
- ↑ "Teva sells 1st generic of Adderall XL in US". Forbes Magazine. Associated Press. 2 April 2009. Archived from the original on 9 April 2009. Retrieved 22 April 2009.
- ↑ "Molecular Weight Calculator". Lenntech. Retrieved 19 August 2015.
- 1 2 "Dextroamphetamine Sulfate USP". Mallinckrodt Pharmaceuticals. March 2014. Retrieved 19 August 2015.
- 1 2 "D-amphetamine sulfate". Tocris. 2015. Retrieved 19 August 2015.
- 1 2 "Amphetamine Sulfate USP". Mallinckrodt Pharmaceuticals. March 2014. Retrieved 19 August 2015.
- ↑ "Dextroamphetamine Saccharate". Mallinckrodt Pharmaceuticals. March 2014. Retrieved 19 August 2015.
- ↑ "Amphetamine Aspartate". Mallinckrodt Pharmaceuticals. March 2014. Retrieved 19 August 2015.
- ↑ "Vyvanse Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. May 2017. pp. 17–21. Retrieved 10 July 2017.
- ↑ "Controlled Drugs and Substances Act (S.C. 1996, c. 19)". 25 April 2017. Archived from the original on 3 April 2011. Retrieved 23 May 2013.
- ↑ "Importing or Bringing Medication into Japan for Personal Use". Japan Ministry of Health, Labour and Welfare. Archived from the original on 13 July 2013. Retrieved 13 October 2013.
- ↑ "Caught in the Dragnet". 2 August 2002. Archived from the original on 19 November 2018. Retrieved 18 November 2018.
- ↑ "Thailand Law" (PDF). Government of Thailand. Archived from the original (PDF) on 8 March 2014. Retrieved 23 May 2013.
- ↑ "Class A, B and C drugs". Home Office, Government of the United Kingdom. Archived from the original on 4 August 2007. Retrieved 23 July 2007.
- ↑ Substance Abuse and Mental Health Services Administration. "Trends in Methamphetamine/Amphetamine Admissions to Treatment: 1993–2003". The Drug and Alcohol Services Information System (DASIS) Report. United States Department of Health and Human Services. Archived from the original on 5 March 2013. Retrieved 6 May 2016.
- ↑ United Nations Office on Drugs and Crime (2007). Preventing Amphetamine-type Stimulant Use Among Young People: A Policy and Programming Guide (PDF). New York: United Nations. ISBN 978-92-1-148223-2. Archived (PDF) from the original on 16 October 2013. Retrieved 7 February 2012.
- ↑ International Narcotics Control Board. "List of psychotropic substances under international control" (PDF). Vienna: United Nations. Archived from the original (PDF) on 5 December 2005. Retrieved 19 November 2005.
External links
| Identifiers: |
|---|
- "Amphetamine". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 17 July 2020. Retrieved 7 April 2020.
- "Amphetamine sulfate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 7 April 2020. Retrieved 7 April 2020.
- "Amphetamine aspartate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 7 April 2020. Retrieved 7 April 2020.
- "Dextroamphetamine saccharate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 7 April 2020. Retrieved 7 April 2020.
- "Amphetamine". MedlinePlus. Archived from the original on 6 May 2020. Retrieved 7 April 2020.