Vagus nerve
| Vagus nerve | |
|---|---|
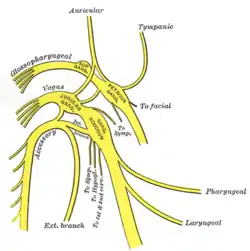 Plan of the upper portions of the glossopharyngeal, vagus, and accessory nerves. | |
 Course and distribution of the glossopharyngeal, vagus, and accessory nerves. | |
| Details | |
| Innervates | Levator veli palatini, Salpingopharyngeus, Palatoglossus, Palatopharyngeus, Superior pharyngeal constrictor, Middle pharyngeal constrictor, Inferior pharyngeal constrictor, viscera |
| Identifiers | |
| Latin | nervus vagus |
| MeSH | D014630 |
| NeuroNames | 702 |
| TA98 | A14.2.01.153 |
| TA2 | 6332 |
| FMA | 5731 |
| Anatomical terms of neuroanatomy | |
| Cranial nerves |
|---|
|
The vagus nerve, historically cited as the pneumogastric nerve, is the tenth cranial nerve or CN X, and interfaces with the parasympathetic control of the heart, lungs, and digestive tract. It actually comprises two nerves—the left and right vagus nerves—but they are typically referred to collectively in the singular. The vagus is the longest nerve of the autonomic nervous system in the human body and comprises sensory and motor fibers. The sensory fibers originate from neurons of the nodose ganglion, whereas the motor fibers come from neurons of the dorsal motor nucleus of the vagus and the nucleus ambiguus.[1]
Structure
Upon leaving the medulla oblongata between the olive and the inferior cerebellar peduncle, the vagus nerve extends through the jugular foramen, then passes into the carotid sheath between the internal carotid artery and the internal jugular vein down to the neck, chest, and abdomen, where it contributes to the innervation of the viscera, reaching all the way to the colon. Besides giving some output to various organs, the vagus nerve comprises between 80% and 90% of afferent nerves mostly conveying sensory information about the state of the body's organs to the central nervous system.[2] The right and left vagus nerves descend from the cranial vault through the jugular foramina, penetrating the carotid sheath between the internal and external carotid arteries, then passing posterolateral to the common carotid artery. The cell bodies of visceral afferent fibers of the vagus nerve are located bilaterally in the inferior ganglion of the vagus nerve (nodose ganglia).
The right vagus nerve gives rise to the right recurrent laryngeal nerve, which hooks around the right subclavian artery and ascends into the neck between the trachea and esophagus. The right vagus then crosses anterior to the right subclavian artery, runs posterior to the superior vena cava, descends posterior to the right main bronchus, and contributes to cardiac, pulmonary, and esophageal plexuses. It forms the posterior vagal trunk at the lower part of the esophagus and enters the diaphragm through the esophageal hiatus.
The left vagus nerve enters the thorax between left common carotid artery and left subclavian artery and descends on the aortic arch. It gives rise to the left recurrent laryngeal nerve, which hooks around the aortic arch to the left of the ligamentum arteriosum and ascends between the trachea and esophagus. The left vagus further gives off thoracic cardiac branches, breaks up into the pulmonary plexus, continues into the esophageal plexus, and enters the abdomen as the anterior vagal trunk in the esophageal hiatus of the diaphragm.
Branches
- Pharyngeal nerve
- Superior laryngeal nerve
- Superior cervical cardiac branches of vagus nerve
- Inferior cervical cardiac branch
- Recurrent laryngeal nerve
- Thoracic cardiac branches
- Branches to the pulmonary plexus
- Branches to the esophageal plexus
- Anterior vagal trunk
- Posterior vagal trunk
- Hering–Breuer reflex in alveoli[3]
The vagus runs parallel to the common carotid artery and internal jugular vein inside the carotid sheath.
Nuclei
The vagus nerve includes axons which emerge from or converge onto four nuclei of the medulla:
- The dorsal nucleus of vagus nerve – which sends parasympathetic output to the viscera, especially the intestines
- The nucleus ambiguus – which gives rise to the branchial efferent motor fibers of the vagus nerve and preganglionic parasympathetic neurons that innervate the heart
- The solitary nucleus – which receives afferent taste information and primary afferents from visceral organs
- The spinal trigeminal nucleus – which receives information about deep/crude touch, pain, and temperature of the outer ear, the dura of the posterior cranial fossa and the mucosa of the larynx
Development
The motor division of the vagus nerve is derived from the basal plate of the embryonic medulla oblongata, while the sensory division originates from the cranial neural crest.
Function
The vagus nerve supplies motor parasympathetic fibers to all the organs (except the adrenal glands), from the neck down to the second segment of the transverse colon. The vagus also controls a few skeletal muscles, including:
- Cricothyroid muscle
- Levator veli palatini muscle
- Salpingopharyngeus muscle
- Palatoglossus muscle
- Palatopharyngeus muscle
- Superior, middle and inferior pharyngeal constrictors
- Muscles of the larynx (speech).
This means that the vagus nerve is responsible for such varied tasks as heart rate, gastrointestinal peristalsis, sweating, and quite a few muscle movements in the mouth, including speech (via the recurrent laryngeal nerve). It also has some afferent fibers that innervate the inner (canal) portion of the outer ear (via the auricular branch, also known as Arnold's or Alderman's nerve) and part of the meninges.[4]
Efferent vagus nerve fibers innervating the pharynx and back of the throat are responsible for the gag reflex. In addition, 5-HT3 receptor-mediated afferent vagus stimulation in the gut due to gastroenteritis is a cause of vomiting.[5] Stimulation of the vagus nerve in the cervix uteri (as in some medical procedures) can lead to a vasovagal response.
The vagus nerve also plays a role in satiation following food consumption.[6] Knocking out vagal nerve receptors has been shown to cause hyperphagia (greatly increased food intake).[7]
Cardiac effects

Parasympathetic innervation of the heart is partially controlled by the vagus nerve and is shared by the thoracic ganglia. Vagal and spinal ganglionic nerves mediate the lowering of the heart rate. The right vagus branch innervates the sinoatrial node. In healthy people, parasympathetic tone from these sources is well-matched to sympathetic tone. Hyperstimulation of parasympathetic influence promotes bradyarrhythmias. When hyperstimulated, the left vagal branch predisposes the heart to conduction block at the atrioventricular node.
At this location, neuroscientist Otto Loewi first demonstrated that nerves secrete substances called neurotransmitters, which have effects on receptors in target tissues. In his experiment, Loewi electrically stimulated the vagus nerve of a frog heart, which slowed the heart. Then he took the fluid from the heart and transferred it to a second frog heart without a vagus nerve. The second heart slowed without electrical stimulation. Loewi described the substance released by the vagus nerve as vagusstoff, which was later found to be acetylcholine. Drugs that inhibit the muscarinic receptors (anticholinergics) such as atropine and scopolamine, are called vagolytic because they inhibit the action of the vagus nerve on the heart, gastrointestinal tract, and other organs. Anticholinergic drugs increase heart rate and are used to treat bradycardia.
Urogenital and hormonal effects
Excessive activation of the vagal nerve during emotional stress, which is a parasympathetic overcompensation for a strong sympathetic nervous system response associated with stress, can also cause vasovagal syncope due to a sudden drop in cardiac output, causing cerebral hypoperfusion. Vasovagal syncope affects young children and women more than other groups. It can also lead to temporary loss of bladder control under moments of extreme fear.
Research has shown that women having had complete spinal cord injury can experience orgasms through the vagus nerve, which can go from the uterus and cervix to the brain.[8][9]
Insulin signaling activates the adenosine triphosphate (ATP)-sensitive potassium (KATP) channels in the arcuate nucleus, decreases AgRP release, and through the vagus nerve, leads to decreased glucose production by the liver by decreasing gluconeogenic enzymes: Phosphoenolpyruvate carboxykinase, Glucose 6-phosphatase.[10][11]
Clinical significance
Stimulation
Vagus nerve stimulation (VNS) therapy using a neurostimulator implanted in the chest is a treatment used since 1997 to control seizures in epilepsy patients and has been approved for treating drug-resistant cases of clinical depression.[12] A non-invasive VNS device that stimulates an afferent branch of the vagus nerve is also being developed and will soon undergo trials.[13]
Clinical trials have started in Antwerp, Belgium, using VNS for the treatment of tonal tinnitus[14] after a breakthrough study published in early 2011 by researchers at the University of Texas – Dallas showed successful tinnitus-suppression in rats when tones were paired with brief pulses of stimulation of the vagus nerve.[15][16]
VNS may also be achieved by one of the vagal maneuvers: holding the breath for 20–60 seconds, dipping the face in cold water, coughing, or tensing the stomach muscles as if to bear down to have a bowel movement.[17] Patients with supraventricular tachycardia,[17] atrial fibrillation, and other illnesses may be trained to perform vagal maneuvers (or find one or more on their own).
Vagus nerve blocking (VBLOC) therapy is similar to VNS but used only during the day. In a six-month open-label trial involving three medical centers in Australia, Mexico, and Norway, vagus nerve blocking helped 31 obese participants lose an average of nearly 15 percent of their excess weight. As of 2008, a year-long 300 participant double-blind, phase II trial had begun.[18]
Vagotomy
Vagotomy (cutting of the vagus nerve) is a now obsolete therapy that was performed for peptic ulcer disease. Vagotomy is currently being researched as a less invasive alternative weight-loss procedure to gastric bypass surgery.[19] The procedure curbs the feeling of hunger and is sometimes performed in conjunction with putting bands on patients' stomachs, resulting in an average of 43% of excess weight loss at six months with diet and exercise.[20]
One serious side effect of vagotomy is a vitamin B12 deficiency later in life – perhaps after about 10 years – that is similar to pernicious anemia. The vagus normally stimulates the stomach's parietal cells to secrete acid and intrinsic factor. Intrinsic factor is needed to absorb vitamin B12 from food. The vagotomy reduces this secretion and ultimately leads to deficiency, which, if left untreated, causes nerve damage, tiredness, dementia, paranoia, and ultimately death.[21]
Researchers from Aarhus University and Aarhus University Hospital have demonstrated that vagotomy prevents (halves the risk of) the development of Parkinson's disease, suggesting that Parkinson's disease begins in the gastrointestinal tract and spreads via the vagus nerve to the brain.[22] Or giving further evidence to the theory that dysregulated environmental stimuli, such as that received by the vagus nerve from the gut, may have a negative effect on the dopamine reward system of the substantia nigra, thereby causing Parkinson's disease.[23]
Chagas disease
The neuropathy in Chagas disease spreads in part via the major parasympathetic branches of the vagus nerve.[24]
Sensory neuropathy
The hypersensitivity of vagal afferent nerves causes refractory or idiopathic cough.
Arnold's nerve ear-cough reflex though uncommon is a manifestation of a vagal sensory neuropathy and this is the cause of a refractory chronic cough that can be treated with gabapentin. The cough is triggered by mechanical stimulation of the external auditory meatus and accompanied by other neuropathic features such as throat irritation (laryngeal paresthesia) and cough triggered by exposure to nontussive triggers such as cold air and eating (termed allotussia). These features suggest a neuropathic origin to the cough.[25]
History
Etymology
The Latin word vagus means literally "wandering" (the words vagrant, vagabond, vague, and divagation come from the same root). Sometimes the right and left branches together are spoken of in the plural and are thus called vagi (/ˈveɪdʒaɪ/ VAY-jy). The vagus was also historically called the pneumogastric nerve since it innervates both the lungs and the stomach.
Additional illustrations
 Inferior view of the human brain, with the cranial nerves labeled.
Inferior view of the human brain, with the cranial nerves labeled. Section of the neck at about the level of the sixth cervical vertebra
Section of the neck at about the level of the sixth cervical vertebra Transverse section of thorax, showing relations of pulmonary artery
Transverse section of thorax, showing relations of pulmonary artery The arch of the aorta, and its branches
The arch of the aorta, and its branches Dura mater and its processes exposed by removing part of the right half of the skull, and the brain
Dura mater and its processes exposed by removing part of the right half of the skull, and the brain The tracheobronchial lymph glands
The tracheobronchial lymph glands Section of the medulla oblongata at about the middle of the olive
Section of the medulla oblongata at about the middle of the olive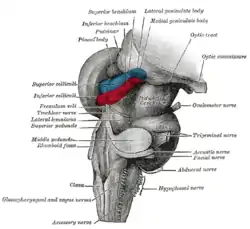 Hind- and mid-brains; postero-lateral view
Hind- and mid-brains; postero-lateral view Upper part of medulla spinalis and hind- and mid-brains; posterior aspect, exposed in situ
Upper part of medulla spinalis and hind- and mid-brains; posterior aspect, exposed in situ The right sympathetic chain and its connections with the thoracic, abdominal, and pelvic plexuses
The right sympathetic chain and its connections with the thoracic, abdominal, and pelvic plexuses The celiac ganglia with the sympathetic plexuses of the abdominal viscera radiating from the ganglia
The celiac ganglia with the sympathetic plexuses of the abdominal viscera radiating from the ganglia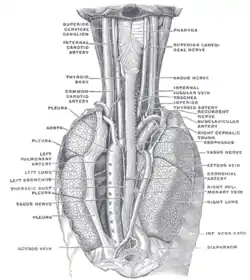 The position and relation of the esophagus in the cervical region and in the posterior mediastinum, seen from behind
The position and relation of the esophagus in the cervical region and in the posterior mediastinum, seen from behind The thyroid gland and its relations
The thyroid gland and its relations The thymus of a full-term fetus, exposed in situ
The thymus of a full-term fetus, exposed in situ Deep dissection of vagus nerve
Deep dissection of vagus nerve Vagus nerve – dissection
Vagus nerve – dissection
See also
- Porphyria – A rare disorder can cause seizures and damage to the vagal nerve.
- Vagovagal reflex
- Inflammatory reflex
- Vagus ganglion
- Vagus nerve stimulation
- Vagusstoff
References
- ↑ Walker, H. Kenneth (1990). Cranial Nerve XI: The Spinal Accessory Nerve. NCBI Bookshelf. Butterworths. ISBN 9780409900774. PMID 21250228. Retrieved 30 May 2019.
- ↑ Berthoud HR, Neuhuber WL (December 2000). "Functional and chemical anatomy of the afferent vagal system". Autonomic Neuroscience. 85 (1–3): 1–17. doi:10.1016/S1566-0702(00)00215-0. PMID 11189015. S2CID 30221339.
- ↑ Dutschmann M, Bautista TG, Mörschel M, Dick TE (May 2014). "Learning to breathe: habituation of Hering-Breuer inflation reflex emerges with postnatal brainstem maturation". Respiratory Physiology & Neurobiology. 195: 44–9. doi:10.1016/j.resp.2014.02.009. PMC 4111629. PMID 24566392.
- ↑ Eljamel, Sam (2011). Problem Based Neurosurgery. p. 66. doi:10.1142/7830. ISBN 978-981-4317-07-8. S2CID 78277439.
- ↑ Mandal, Ananya (25 September 2013). "Vomiting Mechanism". News Medical. Archived from the original on 4 January 2015. Retrieved 27 June 2015.
- ↑ Berthoud HR (August 2008). "The vagus nerve, food intake and obesity". Regulatory Peptides. 149 (1–3): 15–25. doi:10.1016/j.regpep.2007.08.024. PMC 2597723. PMID 18482776.
- ↑ de Lartigue G, Ronveaux CC, Raybould HE (September 2014). "Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity". Molecular Metabolism. 3 (6): 595–607. doi:10.1016/j.molmet.2014.06.003. PMC 4142400. PMID 25161883.
- ↑ "Exploring the Mind-Body Orgasm". Wired. 10 January 2007. Archived from the original on 19 September 2015.
- ↑ Komisaruk BR, Whipple B, Crawford A, Liu WC, Kalnin A, Mosier K (October 2004). "Brain activation during vaginocervical self-stimulation and orgasm in women with complete spinal cord injury: fMRI evidence of mediation by the vagus nerves". Brain Research. 1024 (1–2): 77–88. doi:10.1016/j.brainres.2004.07.029. PMID 15451368. S2CID 9202518.
- ↑ Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L (April 2005). "Hypothalamic K(ATP) channels control hepatic glucose production". Nature. 434 (7036): 1026–31. Bibcode:2005Natur.434.1026P. doi:10.1038/nature03439. PMID 15846348. S2CID 4414624.
- ↑ Pagotto U (November 2009). "Where does insulin resistance start? The brain". Diabetes Care. 32 Suppl 2 (2): S174-7. doi:10.2337/dc09-S305. PMC 2811464. PMID 19875547.
- ↑ Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, George MS, Charney DS, Brannan SK (July 2006). "VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms". Neuropsychopharmacology. 31 (7): 1345–1355. doi:10.1038/sj.npp.1301082. PMID 16641939.
- ↑ Eisenstein, M. (October 2013). "Neurodevice startups target peripheral nervous system". Nature Biotechnology. 31 (10): 865–866. doi:10.1038/nbt1013-865. PMID 24104737. S2CID 205271871.
- ↑ "Feedback on Nexium Side Effects and Usage, page 54". www.askdocweb.com. Archived from the original on 28 December 2017. Retrieved 28 December 2017.
Clinical trials are currently underway in Antwerp, Belgium using VNS for the treatment of tonal tinnitus. This came from a breakthrough published in 2011 by researchers at the University of Texas, Dallas. They found that tinnitus could be successfully suppressed in rats when tones were paired with brief pulses of stimulation of the vagus nerve.
- ↑ "Nerve Stimulation Highly Effective in Battling Tinnitus, Nature Study Reports" (Press release). UT Dallas News. Archived from the original on 24 February 2011. Retrieved 25 March 2011.
- ↑ Tyler R, Cacace A, Stocking C, Tarver B, Engineer N, Martin J, Deshpande A, Stecker N, Pereira M, Kilgard M, Burress C, Pierce D, Rennaker R, Vanneste S (September 2017). "Vagus nerve stimulation paired with tones for the treatment of tinnitus: A prospective randomized double-blind controlled pilot study in humans". Scientific Reports. 7 (1): 11960. Bibcode:2017NatSR...711960T. doi:10.1038/s41598-017-12178-w. PMC 5607328. PMID 28931943.
- 1 2 Vibhuti N., Singh; Gugneja, Monika (22 August 2005). "Supraventricular Tachycardia". eMedicineHealth.com. Archived from the original on 16 December 2008. Retrieved 28 November 2008.
- ↑
"Device blocking stomach nerve signals shows promise in obesity" (Press release). Mayo Clinic. Archived from the original on 8 March 2009.
Dr. Camilleri says a follow-up double-blinded study, which will involve up to 300 patients at multiple medical centers including a limited number from Mayo Clinic, will be important for gauging the device's true effectiveness.
- ↑ Ulcer surgery may help treat obesity – Diet and nutrition. NBC News
- ↑ "Could nerve-snip spur weight loss?". CNN.com. Turner Broadcasting System. Archived from the original on 13 July 2007.
- ↑ The Pernicious Anemia Society Archived 24 July 2010 at the Wayback Machine
- ↑ Parkinson's disease may begin in the gut. Archived 27 June 2015 at the Wayback Machine
- ↑ Liu B, Fang F, Pedersen NL, Tillander A, Ludvigsson JF, Ekbom A, Svenningsson P, Chen H, Wirdefeldt K (May 2017). "Vagotomy and Parkinson disease: A Swedish register-based matched-cohort study". Neurology. 88 (21): 1996–2002. doi:10.1212/WNL.0000000000003961. PMC 5440238. PMID 28446653.
- ↑ Córdova E, Maiolo E, Corti M, Orduña T (April 2010). "Neurological manifestations of Chagas' disease". Neurological Research. 32 (3): 238–44. doi:10.1179/016164110X12644252260637. PMID 20406601. S2CID 206837793.
- ↑ Ryan, Nicole M.; Gibson, Peter G.; Birring, Surinder S. (October 2014). "Arnold's nerve cough reflex: evidence for chronic cough as a sensory vagal neuropathy". Journal of Thoracic Disease. 6 (Suppl 7): S748–S752. doi:10.3978/j.issn.2072-1439.2014.04.22. ISSN 2072-1439. PMC 4222929. PMID 25383210.
External links
| Wikimedia Commons has media related to Nervus vagus. |
- MedEd at Loyola grossanatomy/h_n/cn/cn1/cn10.htm
- "10-1". Cranial Nerves. Yale School of Medicine. Archived from the original on 3 March 2016.
- cranialnerves at The Anatomy Lesson by Wesley Norman (Georgetown University) (X)