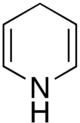
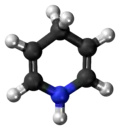
1,4-Dihydropyridine
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,4-Dihydropyridine[1] | |||
| Identifiers | |||
CAS Number |
|||
3D model (JSmol) |
|||
| ChemSpider | |||
| MeSH | 1,4-dihydropyridine | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
C 5H 7N | ||
| Molar mass | 81.1158 g mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
1,4-Dihydropyridine (DHP) is an organic compound with the formula CH2(CH=CH)2NH. The parent compound is uncommon,[2] but derivatives of 1,4-dihydropyridine are important commercially and biologically. The pervasive cofactors NADH and NADPH are derivatives of 1,4-dihydropyridine. 1,4-Dihydropyridine-based drugs are L-type calcium channel blockers, used in the treatment of hypertension. 1,2-Dihydropyridines are also known.[3][4]
Properties and reactions
A recurring feature of 1,4-dihydropyridines is the presence of substituents at the 2- and 6-positions. Dihydropyridines are enamines, which otherwise tend to tautomerize or hydrolyze.
The dominant reaction of dihydropyridines is their ease of oxidation. In the case of dihydropyridines with hydrogen as the substituent on nitrogen, oxidation yields pyridines:
- CH2(CH=CR)2NH → C5H3R2N + H2
The naturally-occurring dihydropyridines NADH and NADPH contain N-alkyl groups. Therefore, their oxidation does not yield pyridine, but N-alkylpyridinium cations:
- CH2(CH=CR)2NR' → C5H3R2NR' + H−
See also

- Dihydropyridine calcium channel blockers
- Hantzsch ester[5]
- Dihydropyridine receptor
References
- ↑ "1,4-dihydropyridine - Compound Summary". Pubchem Compound. USA: National Center for Biotechnology Information. 27 March 2005. Identification and Related Records. Retrieved 1 November 2011.
- ↑ Duburs, Gunãrs; Sausins, Alvils (1988). "Synthesis of 1,4-Dihydropyridines by Cyclocondensation Reactions". Heterocycles. 27: 269. doi:10.3987/REV-87-370.
- ↑ Stout, David M.; Meyers, A. I. (1982). "Recent advances in the chemistry of dihydropyridines". Chemical Reviews. 82 (2): 223–243. doi:10.1021/cr00048a004.
- ↑ Lavilla, Rodolfo (2002). "Recent developments in the chemistry of dihydropyridines". Journal of the Chemical Society, Perkin Transactions 1 (9): 1141–1156. doi:10.1039/B101371H.
- ↑ Cheung, Lawrence L. W.; Styler, Sarah A.; Dicks, Andrew P. (2010). "Rapid and Convenient Synthesis of the 1,4-Dihydropyridine Privileged Structure". Journal of Chemical Education. 87 (6): 628–630. Bibcode:2010JChEd..87..628C. doi:10.1021/ed100171g.
External links
- Dihydropyridines at the US National Library of Medicine Medical Subject Headings (MeSH)