Ehlers–Danlos syndromes
| Ehlers–Danlos syndromes | |
|---|---|
.png.webp) | |
| Individual with EDS displaying skin hyperelasticity | |
| Pronunciation |
|
| Specialty | Medical genetics, rheumatology |
| Symptoms | Overly flexible joints, stretchy skin, abnormal scar formation[1] |
| Complications | Aortic dissection, joint dislocations, osteoarthritis[1] |
| Usual onset | Birth or early childhood[2] |
| Duration | Lifelong[3] |
| Types | Hypermobile, classic, vascular, kyphoscoliosis, arthrochalasia, dermatosparaxis, brittle cornea syndrome, others[4] |
| Causes | Genetic[1] |
| Risk factors | Family history[1] |
| Diagnostic method | Genetic testing, skin biopsy[3] |
| Differential diagnosis | Marfan syndrome, cutis laxa syndrome, familial joint hypermobility syndrome[3] |
| Treatment | Supportive[5] |
| Prognosis | Depends on specific disorder[3] |
| Frequency | 1 in 5,000[1] |
Ehlers–Danlos syndromes (EDS) are a group of rare genetic connective tissue disorders.[1] Symptoms may include loose joints, joint pain, stretchy velvety skin, and abnormal scar formation.[1] These can be noticed at birth or in early childhood.[2] Complications may include aortic dissection, joint dislocations, scoliosis, chronic pain, or early osteoarthritis.[1][3]
EDS occurs due to variations of more than 19 different genes which are present at birth.[1] The specific gene affected determines the type of EDS.[1] Some cases result from a new variation occurring during early development, while others are inherited in an autosomal dominant or recessive manner.[1] Typically, these variations result in defects in the structure or processing of the protein collagen.[1] Diagnosis is often based on symptoms and confirmed with genetic testing or skin biopsy.[3] However, people may initially be misdiagnosed with hypochondriasis, depression, or chronic fatigue syndrome.[3]
There is no known cure.[5] Treatment is supportive in nature.[3] Physical therapy and bracing may help strengthen muscles and support joints.[3] While some forms of EDS result in a normal life expectancy, those that affect blood vessels generally decrease life expectancy.[5]
EDS affects at least one in 5,000 people globally.[1] The prognosis depends on the specific disorder.[3] Excess mobility was first described by Hippocrates in 400 BC.[6] The syndromes are named after two physicians, Edvard Ehlers from Denmark and Henri-Alexandre Danlos from France, who described them at the turn of the 20th century.[7]
Signs and symptoms
This group of disorders affects connective tissues across the body, with symptoms most typically present in the joints, skin, and blood vessels. Effects may range from mildly loose joints to life-threatening cardiovascular complications.[8] Due to the diversity of subtypes within the EDS family, symptoms may vary widely between individuals diagnosed with EDS.
Musculoskeletal
Musculoskeletal symptoms include hyperflexible joints that are unstable and prone to sprain, dislocation, subluxation, and hyperextension.[3][7] There can be an early onset of advanced osteoarthritis,[9] chronic degenerative joint disease,[9] swan-neck deformity of the fingers,[10] and Boutonniere deformity of the fingers. Tearing of tendons or muscles may occur.[11] Deformities of the spine, such as scoliosis (curvature of the spine), kyphosis (a thoracic hump), tethered spinal cord syndrome, and occipitoatlantoaxial hypermobility may also be present.[12] There can also be myalgia (muscle pain) and arthralgia (joint pain),[13] which may be severe and disabling. Trendelenburg's sign is often seen, which means that when standing on one leg, the pelvis drops on the other side.[14] Osgood–Schlatter disease, a painful lump on the knee, is common as well.[15] In infants, walking can be delayed (beyond 18 months of age), and bottom-shuffling instead of crawling occurs.[16]
 Individual with EDS showing hypermobile fingers, including the "swan-neck" malformation on the 2nd–5th digits, and a hypermobile thumb
Individual with EDS showing hypermobile fingers, including the "swan-neck" malformation on the 2nd–5th digits, and a hypermobile thumb Individual with EDS displaying hypermobile thumb
Individual with EDS displaying hypermobile thumb Individual with EDS displaying hypermobile metacarpophalangeal joints
Individual with EDS displaying hypermobile metacarpophalangeal joints Kyphoscoliosis of the back of someone with kyphoscoliosis EDS.
Kyphoscoliosis of the back of someone with kyphoscoliosis EDS.
Skin
The weak connective tissue causes abnormal skin. This may present as stretchy or in other types simply be velvet soft. In all types there is some increased fragility but the degree varies depending on the underlying subtype. Skin may tear and bruises easily, and may heal with abnormal atrophic scars[9] and atrophic scars that look like cigarette paper are a sign seen including in those whose skin might appear otherwise normal.[1][7][17] In some sub-types, although not in the hypermobile subtype, redundant skin folds occur, especially on the eyelids. Redundant skin folds are areas of excess skin lying in folds.[9][18]
Other skin symptoms include molluscoid pseudotumors,[19] especially on pressure points, petechiae,[20] subcutaneous spheroids,[19] livedo reticularis, and piezogenic papules are less common.[21] In vascular EDS, skin can also be thin and translucent. In dermatosparaxis EDS, the skin is extremely fragile and saggy.[1]
 Atrophic scar in a case of EDS
Atrophic scar in a case of EDS.png.webp) Translucent skin in vascular EDS
Translucent skin in vascular EDS Individual with EDS displaying skin hyperelasticity
Individual with EDS displaying skin hyperelasticity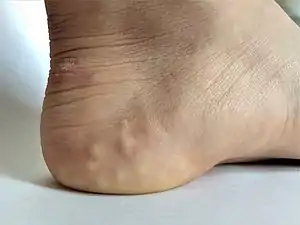 Piezogenic papules on the heel of an individual with hypermobile EDS
Piezogenic papules on the heel of an individual with hypermobile EDS
Cardiovascular
- Thoracic outlet syndrome[22]
- Arterial rupture[7]
- Valvular heart disease, such as mitral valve prolapse, creates an increased risk for infective endocarditis during surgery. This may progress to a life-threatening degree.[23] Heart conduction abnormalities have been found in those with hypermobility form of EDS.[24]
- Dilation and/or rupture (aneurysm) of ascending aorta[25]
- Cardiovascular autonomic dysfunction such as postural orthostatic tachycardia syndrome[26][27]
- Raynaud's phenomenon
- Varicose veins[28]
- Heart murmur
- Heart conduction abnormalities
Other manifestations
- Hiatal hernia[19]
- Gastroesophageal reflux[29]
- Poor gastrointestinal motility[30]
- Dysautonomia[31]
- Gorlin's sign (touch tongue to nose)[32]
- Anal prolapse[19]
- Flat feet
- Tracheobronchomalacia
- Collapsed lung (spontaneous pneumothorax)[9]
- Nerve disorders (carpal tunnel syndrome, acroparesthesia, neuropathy, including small fiber neuropathy)[33]
- Insensitivity to local anesthetics.[34]
- Arnold–Chiari malformation[35]
- Platelet aggregation failure (platelets do not clump together properly)[36]
- Mast cell disorders (including mast cell activation syndrome and mastocytosis)[37]
- Pregnancy complications: increased pain, mild to moderate peripartum bleeding, cervical insufficiency, uterine tearing,[11] or premature rupture of membranes.[38]
- Hearing loss may occur in some types[39]
- Eye: Nearsightedness, retinal tearing and retinal detachment, keratoconus, blue sclera, dry eye, Sjogren's syndrome, lens subluxation, angioid streaks, epicanthal folds, strabismus, corneal scarring, brittle cornea syndrome, cataracts, carotid-cavernous sinus fistulas, macular degeneration[40]
- Cranial vertebral instability: caused by trauma(s) to the head and neck areas such as concussion and whiplash. Ligaments in neck are unable to heal properly, therefore, the neck structure does not have the ability to support the skull, which can then sink into the brain stem blocking the normal flow of cerebrospinal fluid, leading to issues related to the autonomic nervous system failing to work properly.[41]
- Celiac disease may be associated with EDS. Also, it can be misdiagnosed as EDS due to common symptoms, including fatigue, pain, gastrointestinal complaints, or cardiovascular autonomic dysfunction.[23]
 Gorlin's sign in a case of EDS.
Gorlin's sign in a case of EDS. A case of keratoglobus in a case of Brittle cornea syndrome
A case of keratoglobus in a case of Brittle cornea syndrome
Because it is often undiagnosed or misdiagnosed in childhood, some instances of EDS have been mischaracterized as child abuse.[42]
The pain associated with EDS ranges from mild to debilitating.[43]
Genetics

Every type of EDS, except the hypermobile type, can be positively tied to specific genetic variation.
Variations in these genes can cause EDS:[44]
- Collagen primary structure and collagen processing: ADAMTS2, COL1A1, COL1A2, COL3A1, COL5A1, COL5A2
- Collagen folding and collagen cross-linking: PLOD1, FKBP14
- Structure and function of myomatrix: TNXB, COL12A1
- Glycosaminoglycan biosynthesis: B4GALT7, B3GALT6, CHST14, DSE
- Complement pathway: C1R, C1S
- Intracellular processes: SLC39A13, ZNF469, PRDM5
Variations in these genes usually alter the structure, production, or processing of collagen or proteins that interact with collagen. Collagen provides structure and strength to connective tissue. A defect in collagen can weaken connective tissue in the skin, bones, blood vessels, and organs, resulting in the features of the disorder.[1] Inheritance patterns depend on the specific syndrome.
Most forms of EDS are inherited in an autosomal dominant pattern, which means only one of the two copies of the gene in question must be altered to cause a disorder. A few are inherited in an autosomal recessive pattern, which means both copies of the gene must be altered for a person to be affected by a disorder. It can also be an individual (de novo or "sporadic") variation. Sporadic variations occur without any inheritance.[45]
Diagnosis
A diagnosis can be made by an evaluation of medical history and clinical observation. The Beighton criteria are widely used to assess the degree of joint hypermobility. DNA and biochemical studies can help identify affected individuals. Diagnostic tests include collagen gene-variant testing, collagen typing via skin biopsy, echocardiogram, and lysyl hydroxylase or oxidase activity. However, these tests are not able to confirm all cases, especially in instances of an unmapped variation, so clinical evaluation remains important. If multiple individuals in a family are affected, performing prenatal diagnosis may be possible using a DNA information technique known as a linkage study.[46] Knowledge about EDS among all kinds of practitioners is poor.[47][48] Research is ongoing to identify genetic markers for all types.[49]
Classification
In 2017, 13 subtypes of EDS were classified using specific diagnostic criteria.[4] According to The Ehlers-Danlos Society, the syndromes can also be grouped by the symptoms determined by specific gene mutations. Group A disorders are those which affect primary collagen structure and processing. Group B disorders affect collagen folding and crosslinking. Group C are disorders of structure and function of myomatrix. Group D disorders are those that affect glycosaminoglycan biosynthesis. Group E disorders are characterized by defects in the complement pathway. Group F are disorders of intracellular processes, and Group G is considered to be unresolved forms of EDS.[50]
Hypermobile EDS
Hypermobile EDS (formerly categorized as type 3) is mainly characterized by hypermobility that affects both large and small joints. It may lead to frequent joint subluxations (partial dislocations) and dislocations. In general, people with this variant have skin that is soft, smooth, and velvety and bruises easily, as well as chronic muscle and/or bone pain.[4] It affects the skin less than other forms. It has no available genetic test.[23] Hypermobility EDS (hEDS) is the most common of the 19 types of connective tissue disorders. Since there is no known genetic test, providers have to diagnose hEDS based on what they already know about the condition and the physical attributes that the patient shows. Other than the general signs, attributes can include faulty connective tissues throughout the body, musculoskeletal issues, and family history. Along with these general signs and side effects, patients can have trouble healing.[51]
Women who are pregnant should be warned about things such as pre-labor rupture of membranes, drop in blood pressure with anesthesia, precipitate birth (very fast active labor), malposition of bleeding, and more. New mothers with hEDS should pay extra attention to taking care of their new baby. Mothers may have trouble taking care of the baby because of the risk of dropping the baby due to weak connective tissue in arms and legs, falling, postpartum depression (more than the general population), and healing from the birthing process.[52]
Classical EDS
Classical EDS (formerly categorized as type 1) is characterized by extremely elastic skin that is fragile and bruises easily; and hypermobility of the joints. Molluscoid pseudotumors (calcified hematomas that occur over pressure points) and spheroids (cysts that contain fat occurring over forearms and shins) also are seen often. A side complication of the hyperelasticity presented in many cases of EDS makes it more difficult for wounds to close on their own[17]. Sometimes, motor development is delayed and hypotonia occurs.[4] The variation causing this type of EDS is in the genes COL5A2, COL5A1, and less frequently COL1A1. It involves the skin more than hypermobile EDS.[53] In classical EDS there is often large variation in symptom presentation from patient to patient. Because of this variance EDS has often been an under diagnosed disorder.[54] Without genetic testing healthcare professionals may be able to provide a provisional diagnosis based on careful examination of the mouth, skin, and bones. As well as through neurological assessments.[55] The hyperelasticity of skin in EDS patients can be difficult to use in diagnosis because there is no good standardized way to measure and assess the elasticity of the skin. However, hyperelasticity is still a good indicator as something that may point towards EDS along with other symptoms.
A good way to begin the diagnosis process is looking at family history, EDS is an autosomal dominant condition and so is often inherited from family members.[17] Genetic testing remains the most reliable way for an EDS diagnosis to be made.[56] While there is no cure for type 1 EDS, a course of non weight bearing exercising can help with muscular tension which can help correct some of the symptoms of EDS. Anti inflammatory drugs as well as lifestyle changes can help with joint pain. Lifestyle choices should also be made with children that have EDS to try and prevent wounds to the skin. Wearing protective garments can help with this. In the event of a wound often deep stitches are used and left in place for a longer period of time than normal.[17]
Vascular variant of Ehlers–Danlos syndrome
Vascular EDS (formerly categorized as type 4) is identified by skin that is thin, translucent, extremely fragile, and bruises easily. It is also characterized by fragile blood vessels and organs that can easily rupture. Affected people are frequently short, and have thin scalp hair. It also has characteristic facial features including large eyes, an undersized chin, sunken cheeks, a thin nose and lips, and ears without lobes.[57] Joint hypermobility is present, but generally confined to the small joints (fingers, toes). Other common features include club foot, tendon and/or muscle rupture, acrogeria (premature aging of the skin of the hands and feet), early onset varicose veins, pneumothorax (collapse of a lung), recession of the gums, and a decreased amount of fat under the skin.[4] It can be caused by the variations in the COL3A1 gene.[57] Rarely, COL1A1 variations can also cause it.[58]
Kyphoscoliosis EDS
Kyphoscoliosis EDS (formerly categorized as type 6) is associated with severe hypotonia at birth, delayed motor development, progressive scoliosis (present from birth), and scleral fragility. People may also have easy bruising, fragile arteries that are prone to rupture, unusually small corneas, and osteopenia (low bone density). Other common features include a "marfanoid habitus" which is characterized by long, slender fingers (arachnodactyly), unusually long limbs, and a sunken chest (pectus excavatum) or protruding chest (pectus carinatum).[4] It can be caused by variations in the gene PLOD1, or rarely, in the FKBP14 gene.[59]
Arthrochalasia EDS
Arthrochalasia EDS (formerly categorized as types 7A & B) is characterized by severe joint hypermobility and congenital hip dislocation. Other common features include fragile, elastic skin with easy bruising, hypotonia, kyphoscoliosis (kyphosis and scoliosis), and mild osteopenia.[4] Type-I collagen is usually affected. It is very rare, with about 30 cases reported. It is more severe than the hypermobility type. Variations in the genes COL1A1 and COL1A2 cause it.[60]
Dermatosparaxis EDS
Dermatosparaxis EDS (formerly categorized as type 7C) is associated with extremely fragile skin leading to severe bruising and scarring; saggy, redundant skin, especially on the face; hypermobility ranging from mild to serious; and hernias. Variations in the ADAMTS2 gene cause it. It is extremely rare, with around 11 cases reported.[61]
Brittle cornea syndrome
Brittle cornea syndrome is characterized by the progressive thinning of the cornea, early-onset progressive keratoglobus or keratoconus, nearsightedness, hearing loss, and blue sclerae.[4][62] Classic symptoms, such as hypermobile joints and hyperelastic skin, are also seen often.[63] It has two types. Type 1 occurs due to variations in the ZNF469 gene. Type 2 is due to variations in the PRDM5 gene.[62]
Classical-like EDS
Classical-like EDS is characterized by skin hyperextensibility with velvety skin texture and absence of atrophic scarring, generalized joint hypermobility with or without recurrent dislocations (most often shoulder and ankle), and easily bruised skin or spontaneous ecchymoses (discolorations of the skin resulting from bleeding underneath).[4] It can be caused by variations in the TNXB gene.[58]
Spondylodysplastic EDS
Spondylodysplastic EDS is characterized by short stature (progressive in childhood), muscle hypotonia (ranging from severe congenital, to mild later-onset), and bowing of limbs.[4] It can be caused by variations in both copies of the B4GALT7 gene. Other cases can be caused by variations in the B3GALT6 gene. People with variations in this gene can have kyphoscoliosis, tapered fingers, osteoporosis, aortic aneurysma, and problems with the lungs. Other cases can be caused by the SLC39A13 gene. Those with variations in this gene have protuberant eyes, wrinkled palms of the hands, tapering fingers, and distal joint hypermobility.[64]
Musculocontractural EDS
Musculocontractural EDS is characterized by congenital multiple contractures, characteristically adduction-flexion contractures and/or talipes equinovarus (clubfoot), characteristic craniofacial features, which are evident at birth or in early infancy, and skin features such as skin hyperextensibility, bruising, skin fragility with atrophic scars, and increased palmar wrinkling.[4] It can be caused by variations in the CHST14 gene. Some other cases can be caused by variations in the DSE gene.[65]
Myopathic EDS
Myopathic EDS (mEDS) is characterized by three major criteria: congenital muscle hypotonia and/or muscle atrophy that improves with age, proximal joint contractures of the knee, hip, and elbow, and hypermobility of distal joints (ankles, wrists, feet, and hands).[4] There are also four minor criteria that may contribute to a diagnosis of mEDS. This disorder can be inherited through either an autosomal dominant or an autosomal recessive pattern.[50] Molecular testing must be completed to verify that mutations in the COL12A1 gene are present; if not, other collagen-type myopathies should be considered.[50]
Periodontal EDS
Periodontal EDS (pEDS) is an inherited autosomal dominant disorder[50] characterized by four major criteria of severe and intractable periodontitis of early onset (childhood or adolescence), lack of attached gingiva, pretibial plaques, and family history of a first-degree relative who meets clinical criteria.[4] Eight minor criteria may also contribute to the diagnosis of pEDS. Molecular testing may reveal mutations in C1R or C1S genes affecting the C1r protein.[50]
Cardiac-valvular EDS
Cardiac-valvular EDS (cvEDS) is characterized by three major criteria: severe progressive cardiac-valvular problems (affecting aortic and mitral valves), skin problems such as hyperextensibility, atrophic scarring, thin skin, and easy bruising, and joint hypermobility (generalized or restricted to small joints).[4] There are also four minor criteria which may aid in diagnosis of cvEDS.[50] Cardiac-valvular EDS is an autosomal recessive disorder, inherited through variation in both alleles of the gene COL1A2.[66]
History
Until 1997, the classification system for EDS included 10 specific types, and also acknowledged that other extremely rare types existed. At this time, the classification system underwent an overhaul and was reduced to six major types using descriptive titles. Genetic specialists recognize that other types of this condition exist, but have only been documented in single families. Except for hypermobility (type 3), the most common type of all ten types, some of the specific variations involved have been identified and they can be precisely identified by genetic testing; this is valuable due to a great deal of variation in individual cases. However, negative genetic test results do not rule out the diagnosis, since not all of the variations have been discovered; therefore, the clinical presentation is very important.[67]
Forms of EDS in this category may present with soft, mildly stretchable skin, shortened bones, chronic diarrhea, joint hypermobility and dislocation, bladder rupture, or poor wound healing. Inheritance patterns in this group include X-linked recessive, autosomal dominant, and autosomal recessive. Examples of types of related syndromes other than those above reported in the medical literature include:[68]
Differential diagnosis
Several disorders share some characteristics with EDS. For example, in cutis laxa, the skin is loose, hanging, and wrinkled. In EDS, the skin can be pulled away from the body, but is elastic and returns to normal when let go. In Marfan syndrome, the joints are very mobile and similar cardiovascular complications occur. People with EDS tend to have a "marfanoid" appearance (e.g., tall, skinny, long arms and legs, "spidery" fingers). However, physical appearance and features in several types of EDS also have characteristics including short stature, large eyes, and the appearance of a small mouth and chin, due to a small palate. The palate can have a high arch, causing dental crowding. Blood vessels can sometimes be easily seen through translucent skin, especially on the chest. The genetic connective tissue disorder, Loeys–Dietz syndrome, also has symptoms that overlap with EDS.[69]
In the past, Menkes disease, a copper metabolism disorder, was thought to be a form of EDS. People are not uncommonly misdiagnosed with fibromyalgia, bleeding disorders, or other disorders that can mimic EDS symptoms. Because of these similar disorders and complications that can arise from an unmonitored case of EDS, a correct diagnosis is important.[70] Pseudoxanthoma elasticum (PXE) is worth consideration in diagnosis.[71]
Management
There is no known cure for Ehlers–Danlos syndromes and treatment is supportive. Close monitoring of the cardiovascular system, physiotherapy, occupational therapy, and orthopedic instruments (e.g., wheelchairs, bracing, casting) may be helpful. This can help stabilize the joints and prevent injury. Orthopedic instruments are helpful for the prevention of further joint damage, especially for long distances, although individuals are advised not to become dependent on them until other mobility options have been exhausted. People should avoid activities that cause the joint to lock or overextend.[72]
A physician may prescribe casting to stabilize joints. Physicians may refer a person to an orthotist for orthotic treatment (bracing). Physicians may also consult a physical and/or occupational therapist to help strengthen muscles and to teach people how to properly use and preserve their joints.[73][74]
Aquatic therapy promotes muscular development and coordination.[75] With manual therapy, the joint is gently mobilized within the range of motion and/or manipulations.[73][74] If conservative therapy is not helpful, surgical joint repair may be necessary. Medication to decrease pain or manage cardiac, digestive, or other related conditions may be prescribed. To decrease bruising and improve wound healing, some people have responded to vitamin C.[76] Special precautions are often taken by medical care workers because of the sheer number of complications that tend to arise in people with EDS. In vascular EDS, signs of chest or abdominal pain are considered trauma situations.[77]
Cannabinoids and medical marijuana have shown some efficacy in reducing pain levels.[78]
In general, medical intervention is limited to symptomatic therapy. Before pregnancy, people with EDS should have genetic counseling and familiarize themselves with the risks to their own bodies that pregnancy poses. Children with EDS should be provided with information about their disorder so they can understand why they should avoid contact sports and other physically stressful activities. Children should be taught that demonstrating the unusual positions that they can maintain due to loose joints should not be done, as this may cause early degeneration of the joints. Emotional support along with behavioral and psychological therapy can be useful. Support groups can be immensely helpful for people dealing with major lifestyle changes and poor health. Family members, teachers, and friends should be informed about EDS so they can accept and assist the child.[79]
Surgery
The instability of joints, leading to subluxations and joint pain, often requires surgical intervention in people with EDS. Instability of almost all joints can happen, but appears most often in the lower and upper extremities, with the wrist, fingers, shoulder, knee, hip, and ankle being most common.[73]
Common surgical procedures are joint debridement, tendon replacements, capsulorrhaphy, and arthroplasty. After surgery, the degree of stabilization, pain reduction, and people's satisfaction can improve, but surgery does not guarantee an optimal result: affected peoples and surgeons report being dissatisfied with the results. Consensus is that conservative treatment is more effective than surgery,[24] particularly since people have extra risks of surgical complications due to the disease. Three basic surgical problems arise due to EDS: the strength of the tissues is decreased, which makes the tissue less suitable for surgery; the fragility of the blood vessels can cause problems during surgery; and wound healing is often delayed or incomplete.[73] If considering surgical intervention, seeking care from a surgeon with extensive knowledge and experience in treating people with EDS and joint hypermobility issues would be prudent.[80]
Local anesthetics, arterial catheters, and central venous catheters cause a higher risk of bruise formation in people with EDS. Some people with EDS also show a resistance to local anaesthetics.[81] Resistance to lidocaine and bupivacaine is not uncommon, and mepivacaine tends to work better in people with EDS. There are special recommendations for anesthesia in people with EDS.[82] Detailed recommendations for anesthesia and perioperative care of people with EDS should be used to improve safety.[83]
Surgery in people with EDS requires careful tissue handling and a longer immobilization afterward.[84]
Prognosis
The outcome for individuals with EDS depends on the specific type of EDS they have. Symptoms vary in severity, even in the same disorder, and the frequency of complications varies. Some people have negligible symptoms, while others are severely restricted in daily life. Extreme joint instability, chronic musculoskeletal pain, degenerative joint disease, frequent injuries, and spinal deformities may limit mobility. Severe spinal deformities may affect breathing. In the case of extreme joint instability, dislocations may result from simple tasks such as rolling over in bed or turning a doorknob. Secondary conditions such as autonomic dysfunction or cardiovascular problems, occurring in any type, can affect prognosis and quality of life. Severe mobility-related disability is seen more often in hypermobile EDS than in classical EDS or vascular EDS.[85]
Although all types of EDS are potentially life-threatening, most people have a normal lifespan. However, those with blood-vessel fragility have a high risk of fatal complications, including spontaneous arterial rupture, which is the most common cause of sudden death. The median life expectancy in the population with vascular EDS is 48 years.[86]
Epidemiology
Ehlers–Danlos syndromes are inherited disorders estimated to occur in about one in 5,000 births worldwide. Initially, prevalence estimates ranged from one in 250,000 to 500,000 people, but these estimates were soon found to be too low, as more was studied about the disorders, and medical professionals became more adept at diagnosis. EDS may be far more common than the currently accepted estimate due to the wide range of severities with which the disorder presents.[87]
The prevalence of the disorders differs dramatically. The most commonly occurring is hypermobile EDS, followed by classical EDS. The others are very rare. For example, fewer than 10 infants and children with dermatosparaxis EDS have been described worldwide.
Some types of EDS are more common in Ashkenazi Jews. For example, the chance of being a carrier for dermatosparaxis EDS is one in 248 in Ashkenazi Jews, whereas the prevalence of this variation in the general population is one in 2,000.[88]
Society and culture

EDS may have contributed to the virtuoso violinist Niccolò Paganini's skill, as he was able to play wider fingerings than a typical violinist.[89]
Many sideshow performers have EDS. Several of them were billed as the Elastic Skin Man, the India Rubber Man, and Frog Boy. They included such well-known individuals (in their time) as Felix Wehrle, James Morris, and Avery Childs. Two performers with EDS currently hold world records. Contortionist Daniel Browning Smith has hypermobile EDS and holds the current Guinness World Record for the most flexible man as of 2018, while Gary "Stretch" Turner (shown right), sideshow performer in the Circus Of Horrors, has held the current Guinness World Record for the most elastic skin since 1999, for his ability to stretch the skin on his stomach 6.25 inches.[90]
Notable cases
- Actress Cherylee Houston, hypermobile EDS. She uses a wheelchair and was the first full-time disabled actress on Coronation Street.[91]
- Drag queen Yvie Oddly[92]
- Eric the Actor, a regular caller to The Howard Stern Show[93]
- Actress and activist Jameela Jamil, hypermobile EDS.[94] EDS is now part of her body positivity movements.[94]
- Writer and actress Lena Dunham[95]
- Australian singer Sia[96]
- YouTuber and disability rights activist Annie Elainey[97]
- Miss America 2020 Camille Schrier[98]
Other species
Ehlers–Danlos-like syndromes have been shown to be hereditary in Himalayan cats, some domestic shorthair cats,[99] and certain breeds of cattle.[100] It is seen as a sporadic condition in domestic dogs. It has a similar treatment and prognosis. Animals with the condition should not be bred, as the condition can be inherited.[101]
- Animal EDS
 EDS in a dog
EDS in a dog Same dog with EDS
Same dog with EDS EDS in same dog showing an atrophic scar
EDS in same dog showing an atrophic scar
Degenerative suspensory ligament desmitis (DSLD) is a similar condition seen in many breeds of horses.[102] It was originally notated in the Peruvian Paso and thought to be a condition of overwork and older age. However, DSLD is being recognized in all age groups and all activity levels. It has been noted in newborn foals.
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 "Ehlers–Danlos syndrome". Genetics Home Reference. Archived from the original on 8 May 2016. Retrieved 8 May 2016.
- 1 2 Anderson, Bryan E. (2012). The Netter Collection of Medical Illustrations - Integumentary System E-Book (2 ed.). Elsevier Health Sciences. p. 235. ISBN 978-1455726646. Archived from the original on 2017-11-05.
- 1 2 3 4 5 6 7 8 9 10 11 Lawrence EJ (December 2005). "The clinical presentation of Ehlers-Danlos syndrome". Advances in Neonatal Care. 5 (6): 301–14. doi:10.1016/j.adnc.2005.09.006. PMID 16338669.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 "Ehlers-Danlos syndromes". rarediseases.info.nih.gov. 20 April 2017. Archived from the original on 24 September 2017. Retrieved 23 September 2017.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 3 Ferri, Fred F. (2016). Ferri's Netter Patient Advisor. Elsevier Health Sciences. p. 939. ISBN 9780323393249. Archived from the original on 2017-11-05.
- ↑ Beighton, Peter H.; Grahame, Rodney; Bird, Howard A. (2011). Hypermobility of Joints. Springer. p. 1. ISBN 9781848820852. Archived from the original on 2017-11-05.
- 1 2 3 4 Byers PH, Murray ML (November 2012). "Heritable collagen disorders: the paradigm of the Ehlers-Danlos syndrome". The Journal of Investigative Dermatology. 132 (E1): E6-11. doi:10.1038/skinbio.2012.3. PMID 23154631.

- ↑ "Ehlers-Danlos syndrome". Genetic Home Reference. Archived from the original on 8 May 2016. Retrieved 4 April 2018.
- 1 2 3 4 5 "Ehlers–Danlos Syndrome". Mayo Clinic. Archived from the original on 25 June 2012. Retrieved 25 May 2012.
- ↑ Wei DH, Terrono AL (October 2015). "Superficialis Sling (Flexor Digitorum Superficialis Tenodesis) for Swan Neck Reconstruction". The Journal of Hand Surgery. 40 (10): 2068–74. doi:10.1016/j.jhsa.2015.07.018. PMID 26328902.
- 1 2 "Vascular Type-EDS". Ehlers–Danlos Syndrome Network C.A.R.E.S. Inc. Archived from the original on 2012-06-04. Retrieved 2012-05-25.
- ↑ Dordoni C, Ciaccio C, Venturini M, Calzavara-Pinton P, Ritelli M, Colombi M (August 2016). "Further delineation of FKBP14-related Ehlers-Danlos syndrome: A patient with early vascular complications and non-progressive kyphoscoliosis, and literature review" (PDF). American Journal of Medical Genetics. Part A. 170 (8): 2031–8. doi:10.1002/ajmg.a.37728. PMID 27149304. Archived (PDF) from the original on 2019-03-28. Retrieved 2020-08-07.
- ↑ Gedalia A, Press J, Klein M, Buskila D (July 1993). "Joint hypermobility and fibromyalgia in schoolchildren". Annals of the Rheumatic Diseases. 52 (7): 494–6. doi:10.1136/ard.52.7.494. PMC 1005086. PMID 8346976.
- ↑ Dommerholt, Jan (2012-01-27). "CSF Ehlers Danlos Colloquium, Dr Jan Dommerholt". Chiari & Syringomyelia Foundation. Archived from the original on 4 May 2013. Retrieved 10 June 2013.
- ↑ Vigorita, Vincent J; Mintz, Douglas; Ghelman, Bernard (2008). Orthopaedic pathology (2nd ed.). Philadelphia: Lippincott Williams and Wilkins. pp. 5–6. ISBN 978-0781796705.
- ↑ "Ehlers-Danlos syndrome - Diagnosis - Approach". BMJ Best Practice. 13 December 2016. Archived from the original on 19 August 2010. Retrieved 18 August 2017.
- 1 2 3 4 Malfait F, Wenstrup R, De Paepe A (May 2017). Classic Ehlers-Danlos Syndrome (Gene Reviews ed.). University of Washington. Archived from the original on 2020-06-30. Retrieved 2020-08-07.
- ↑ "Ehlers Danlos Syndrome - Morphopedics". morphopedics.wikidot.com. Archived from the original on 2018-06-15. Retrieved 2018-06-15.
- 1 2 3 4 "Classical Type-EDS". Ehlers–Danlos Syndrome Network C.A.R.E.S Inc. Archived from the original on 2012-05-30. Retrieved 2012-05-25.
- ↑ Portable Signs and Symptoms. Lippincott Williams & Wilkins. 2007. p. 465. ISBN 9781582556796. Archived from the original on 2017-11-05.
- ↑ "Piezogenic papules - DermNet New Zealand". www.dermnetnz.org. Archived from the original on 2016-11-26.
- ↑ Ericson WB, Wolman R (March 2017). "Orthopaedic management of the Ehlers-Danlos syndromes". American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 175 (1): 188–194. doi:10.1002/ajmg.c.31551. eISSN 1552-4876. PMID 28192621.

- 1 2 3 Levy HP, Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (1993). Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJ, Stephens K, Amemiya A (eds.). "Hypermobile Ehlers-Danlos Syndrome". Gene Review [Internet]. University of Washington, Seattle. PMID 20301456. Archived from the original on 2017-01-18. Retrieved 2020-08-07.
- 1 2 Camerota F, Castori M, Celletti C, Colotto M, Amato S, Colella A, et al. (July 2014). "Heart rate, conduction and ultrasound abnormalities in adults with joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type". Clinical Rheumatology. 33 (7): 981–7. doi:10.1007/s10067-014-2618-y. PMID 24752348.
- ↑ Wenstrup RJ, et al. (2001). The Ehlers–Danlos Syndromes: Management of Genetic Syndromes. pp. 131–149.
- ↑ Grigoriou E, Boris JR, Dormans JP (February 2015). "Postural orthostatic tachycardia syndrome (POTS): association with Ehlers-Danlos syndrome and orthopaedic considerations". Clinical Orthopaedics and Related Research. 473 (2): 722–8. doi:10.1007/s11999-014-3898-x. eISSN 1528-1132. PMC 4294907. PMID 25156902.
- ↑ Hakim A, O'Callaghan C, De Wandele I, Stiles L, Pocinki A, Rowe P (March 2017). "Cardiovascular autonomic dysfunction in Ehlers-Danlos syndrome-Hypermobile type". American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 175 (1): 168–174. doi:10.1002/ajmg.c.31543. PMID 28160388.

- ↑ Raffetto JD, Khalil RA (April 2008). "Mechanisms of varicose vein formation: valve dysfunction and wall dilation" (PDF). Phlebology. 23 (2): 85–98. doi:10.1258/phleb.2007.007027. PMID 18453484. Archived (PDF) from the original on 2020-07-12. Retrieved 2020-08-07.
- ↑ Zeitoun JD, Lefèvre JH, de Parades V, Séjourné C, Sobhani I, Coffin B, Hamonet C (November 2013). "Functional digestive symptoms and quality of life in patients with Ehlers-Danlos syndromes: results of a national cohort study on 134 patients". PLOS ONE. 8 (11): e80321. Bibcode:2013PLoSO...880321Z. doi:10.1371/journal.pone.0080321. eISSN 1932-6203. PMC 3838387. PMID 24278273.
- ↑ Brockway, Laura (April 2016). "Gastrointestinal manifestations of Ehlers–Danlos syndrome (hypermobility type)". Ehlers–Danlos Support UK. Archived from the original on 2016-11-14.
- ↑ "Ehlers–Danlos Syndrome". Underlying Causes of Dysautonomia. Dysautonomia International. 2012. Archived from the original on 2014-12-18.
- ↑ Létourneau Y, Pérusse R, Buithieu H (June 2001). "Oral manifestations of Ehlers-Danlos syndrome". Journal. 67 (6): 330–4. PMID 11450296. Archived from the original on 2016-12-15.
- ↑ "Ehlers–Danlos syndrome: Definition from". Answers.com. Archived from the original on 2014-03-06. Retrieved 2014-02-27.
- ↑ Arendt-Nielsen, Lars. "Patients Suffering from Ehlers Danlos Syndrome Type III Do Not Respond to Local Anesthetics". Archived from the original on 2015-04-05.
- ↑ Castori M, Voermans NC (October 2014). "Neurological manifestations of Ehlers-Danlos syndrome(s): A review". Iranian Journal of Neurology. 13 (4): 190–208. PMC 4300794. PMID 25632331.
{{cite journal}}: CS1 maint: url-status (link) - ↑ MedlinePlus Encyclopedia: Ehlers-Danlos syndrome
- ↑ Seneviratne SL, Maitland A, Afrin L (March 2017). "Mast cell disorders in Ehlers-Danlos syndrome". American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 175 (1): 226–236. doi:10.1002/ajmg.c.31555. PMID 28261938.
- ↑ Lind J, Wallenburg HC (April 2002). "Pregnancy and the Ehlers-Danlos syndrome: a retrospective study in a Dutch population". Acta Obstetricia et Gynecologica Scandinavica. 81 (4): 293–300. doi:10.1034/j.1600-0412.2002.810403.x. PMID 11952457. Archived from the original on 2020-07-11. Retrieved 2020-08-07.

- ↑ "Ehlers Danlos Syndromes". NORD (National Organization for Rare Disorders). Archived from the original on 11 November 2019. Retrieved 11 November 2019.
- ↑ "EHLERS-DANLOS SYNDROME – The Role of Collagen in the Eye – Information". Archived from the original on 2019-07-06. Retrieved 2019-07-06.
- ↑ Henderson, Fraser (2015). "Indices of Cranio-vertebral Instability". Funded Research. Chiari & Syringomyelia Foundation. Archived from the original on 2016-09-16.
- ↑ Santschi, Darrell R. (April 3, 2008). "Redlands mother stung by untrue suspicions presses for accountability in child abuse inquiries". The Press Enterprise. Archived from the original on February 28, 2009.
- ↑ Chopra P, Tinkle B, Hamonet C, Brock I, Gompel A, Bulbena A, Francomano C (March 2017). "Pain management in the Ehlers-Danlos syndromes". American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 175 (1): 212–219. doi:10.1002/ajmg.c.31554. PMID 28186390.

- ↑ Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, et al. (March 2017). "The 2017 international classification of the Ehlers-Danlos syndromes". American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 175 (1): 8–26. doi:10.1002/ajmg.c.31552. PMID 28306229.

- ↑ "EDS Types | The Ehlers Danlos Society". The Ehlers Danlos Society. Archived from the original on 2017-06-24. Retrieved 2017-05-22.
- ↑ Sobey G (January 2015). "Ehlers-Danlos syndrome: how to diagnose and when to perform genetic tests". Archives of Disease in Childhood. 100 (1): 57–61. doi:10.1136/archdischild-2013-304822. PMID 24994860. Archived from the original on 2020-05-28. Retrieved 2020-08-07.

- ↑ Ross J, Grahame R (January 2011). "Joint hypermobility syndrome". BMJ. 342: c7167. doi:10.1136/bmj.c7167. PMID 21252103.
- ↑ Castori M (2012). "Ehlers-danlos syndrome, hypermobility type: an underdiagnosed hereditary connective tissue disorder with mucocutaneous, articular, and systemic manifestations". ISRN Dermatology. 2012: 751768. doi:10.5402/2012/751768. PMC 3512326. PMID 23227356.
- ↑ "The Types of EDS". The Ehlers Danlos Society. Archived from the original on 2020-04-21. Retrieved 2018-10-17.
- 1 2 3 4 5 6 "The Types of EDS". The Ehlers Danlos Society. Archived from the original on 2020-04-21. Retrieved 2019-11-06.
- ↑ Carter, Kane. "Hypermobile EDS and Hypermobility Spectrum Disorders". Ehlers-Danlos Support UK. Archived from the original on 2020-09-16.
- ↑ "Pregnancy, birth, feeding and hypermobile Ehlers-Danlos syndrome / hypermobility spectrum disorders – The Ehlers-Danlos Support UK". Archived from the original on 2020-01-30. Retrieved 2019-11-22.
- ↑ Malfait F, Wenstrup R, De Paepe A (1993). Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJ, Stephens K, Amemiya A (eds.). Classic Ehlers-Danlos Syndrome. GeneReviews. University of Washington, Seattle. PMID 20301422. Archived from the original on 2020-06-30. Retrieved 2019-06-03.
- ↑ Kapferer-Seebacher I, Lundberg P, Malfait F, Zschocke J (November 2017). "Periodontal manifestations of Ehlers-Danlos syndromes: A systematic review". Journal of Clinical Periodontology. 44 (11): 1088–1100. doi:10.1111/jcpe.12807. PMID 28836281.
- ↑ Castori M (2012). "Ehlers-danlos syndrome, hypermobility type: an underdiagnosed hereditary connective tissue disorder with mucocutaneous, articular, and systemic manifestations". ISRN Dermatology. 2012: 751768. doi:10.5402/2012/751768. PMC 3512326. PMID 23227356.
- ↑ Rakhmanov Y, Maltese PE, Bruson A, Castori M, Beccari T, Dundar M, Bertelli M (2018-09-01). "Genetic testing for vascular Ehlers-Danlos syndrome and other variants with fragility of the middle arteries". The EuroBiotech Journal. 2 (s1): 42–44. doi:10.2478/ebtj-2018-0034. ISSN 2564-615X. Archived from the original on 2016-08-27. Retrieved 2020-08-07.
- 1 2 Eagleton MJ (December 2016). "Arterial complications of vascular Ehlers-Danlos syndrome". Journal of Vascular Surgery. 64 (6): 1869–1880. doi:10.1016/j.jvs.2016.06.120. PMID 27687326. Archived from the original on 2021-08-28. Retrieved 2020-08-07.

- 1 2 Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, et al. (March 2017). "The 2017 international classification of the Ehlers-Danlos syndromes". American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 175 (1): 8–26. doi:10.1002/ajmg.c.31552. PMID 28306229.

- ↑ "Kyphoscoliotic Ehlers-Danlos syndrome | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". rarediseases.info.nih.gov. Archived from the original on 2019-06-03. Retrieved 2019-06-03.
- ↑ Klaassens M, Reinstein E, Hilhorst-Hofstee Y, Schrander JJ, Malfait F, Staal H, et al. (August 2012). "Ehlers-Danlos arthrochalasia type (VIIA-B)--expanding the phenotype: from prenatal life through adulthood". Clinical Genetics. 82 (2): 121–30. doi:10.1111/j.1399-0004.2011.01758.x. PMC 4026000. PMID 21801164.
- ↑ "Dermatosparaxis Ehlers-Danlos syndrome | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". rarediseases.info.nih.gov. Archived from the original on 2019-06-03. Retrieved 2019-06-03.
- 1 2 "Brittle cornea syndrome | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". rarediseases.info.nih.gov. Archived from the original on 2019-06-03. Retrieved 2019-06-03.
- ↑ "OMIM Entry - # 614170 - BRITTLE CORNEA SYNDROME 2; BCS2". www.omim.org. Archived from the original on 2019-06-03. Retrieved 2018-06-18.
- ↑ "Spondylodysplastic Ehlers-Danlos syndrome | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". rarediseases.info.nih.gov. Archived from the original on 2020-07-08. Retrieved 2019-09-22.
- ↑ "Ehlers-Danlos syndrome, musculocontractural type - Conditions - GTR - NCBI". www.ncbi.nlm.nih.gov. Archived from the original on 2019-09-27. Retrieved 2019-09-22.
- ↑ Guarnieri V, Morlino S, Di Stolfo G, Mastroianno S, Mazza T, Castori M (May 2019). "Cardiac valvular Ehlers-Danlos syndrome is a well-defined condition due to recessive null variants in COL1A2". American Journal of Medical Genetics. Part A. 179 (5): 846–851. doi:10.1002/ajmg.a.61100. PMID 30821104.
- ↑ "What is EDS | The Ehlers–Danlos National Foundation". www.ednf.org. Archived from the original on 2016-04-26. Retrieved 2016-01-06.
- ↑ "OMIM Entry Search - ehlers-danlos syndrome". www.omim.org. Archived from the original on 2020-07-08. Retrieved 2019-04-27.
- ↑ "Differential Diagnosis". www.loeysdietz.org. Archived from the original on 2017-06-23.
- ↑ "Ehlers–Danlos Syndrome". Rarediseases.about.com. 2006-05-25. Archived from the original on 2014-04-12. Retrieved 2014-02-27.
- ↑ "Pseudoxanthoma elasticum". Genetics Home Reference. Archived from the original on 2018-04-18. Retrieved 2018-04-17.
- ↑ "Physiotherapy and self-management – The Ehlers-Danlos Support UK". www.ehlers-danlos.org. Archived from the original on 2019-12-08. Retrieved 2018-04-17.
- 1 2 3 4 Rombaut L, Malfait F, De Wandele I, Cools A, Thijs Y, De Paepe A, Calders P (July 2011). "Medication, surgery, and physiotherapy among patients with the hypermobility type of Ehlers-Danlos syndrome". Archives of Physical Medicine and Rehabilitation. 92 (7): 1106–12. doi:10.1016/j.apmr.2011.01.016. PMID 21636074. Archived from the original on 2021-08-28. Retrieved 2020-08-07.
- 1 2 Woerdeman LA, Ritt MJ, Meijer B, Maas M (2000). "Wrist problems in patients with Ehlers–Danlos syndrome". European Journal of Plastic Surgery. 23 (4): 208–210. doi:10.1007/s002380050252. Archived from the original on 2021-08-28. Retrieved 2020-08-07.
- ↑ Callewaert B, Malfait F, Loeys B, De Paepe A (March 2008). "Ehlers-Danlos syndromes and Marfan syndrome". Best Practice & Research. Clinical Rheumatology. 22 (1): 165–89. doi:10.1016/j.berh.2007.12.005. PMID 18328988.
- ↑ Genetics of Ehlers–Danlos Syndrome~treatment at eMedicine
- ↑ "Vascular (VEDS) Emergency Information | The Ehlers Danlos Society". The Ehlers Danlos Society. Archived from the original on 2018-04-18. Retrieved 2018-04-17.
- ↑ "Topics in Pain Management: pain management in patients with hyper mobility disorders: frequently missed causes of chronic pain" (PDF). Topics in Pain Management. Archived (PDF) from the original on 2021-06-25. Retrieved 2020-08-07.
- ↑ Giroux CM, Corkett JK, Carter LM (2016). "The Academic and Psychosocial Impacts of Ehlers-Danlos Syndrome on Postsecondary Students: An Integrative Review of the Literature" (PDF). Journal of Postsecondary Education and Disability. 29 (4): 414. Archived (PDF) from the original on 2021-03-02. Retrieved 2020-08-07.
- ↑ Wiesmann T, Castori M, Malfait F, Wulf H (July 2014). "Recommendations for anesthesia and perioperative management in patients with Ehlers-Danlos syndrome(s)". Orphanet Journal of Rare Diseases. 9: 109. doi:10.1186/s13023-014-0109-5. PMC 4223622. PMID 25053156.
- ↑ Parapia LA, Jackson C (April 2008). "Ehlers-Danlos syndrome--a historical review". British Journal of Haematology. 141 (1): 32–5. doi:10.1111/j.1365-2141.2008.06994.x. PMID 18324963. S2CID 7809153.

- ↑ OrphanAnesthesia Guidelines
- ↑ Wiesmann T, Castori M, Malfait F, Wulf H (July 2014). "Recommendations for anesthesia and perioperative management in patients with Ehlers-Danlos syndrome(s)". Orphanet Journal of Rare Diseases. 9: 109. doi:10.1186/s13023-014-0109-5. PMC 4223622. PMID 25053156.
- ↑ Shirley ED, Demaio M, Bodurtha J (September 2012). "Ehlers-danlos syndrome in orthopaedics: etiology, diagnosis, and treatment implications". Sports Health. 4 (5): 394–403. doi:10.1177/1941738112452385. PMC 3435946. PMID 23016112.
- ↑ "What are the Ehlers-Danlos Syndromes?". The Ehlers Danlos Society. Archived from the original on 2019-09-11. Retrieved 2019-09-10.
- ↑ Pepin M, Schwarze U, Superti-Furga A, Byers PH (March 2000). "Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type". The New England Journal of Medicine. 342 (10): 673–80. CiteSeerX 10.1.1.603.1293. doi:10.1056/NEJM200003093421001. PMID 10706896.

- ↑ "Ehlers–Danlos Syndrome: Epidemiology". Medscape.com. Archived from the original on 2013-04-24. Retrieved 2014-02-27.
- ↑ "Ehlers-Danlos syndrome type VIIc". geneaware.clinical.bcm.edu. Archived from the original on 2017-08-14. Retrieved 2017-07-24.
- ↑ Yücel D (January 1995). "Was Paganini born with Ehlers-Danlos syndrome phenotype 4 or 3?". Clinical Chemistry. 41 (1): 124–5. doi:10.1093/clinchem/41.1.124. PMID 7813066. Archived from the original on 2021-08-28. Retrieved 2020-08-07.

- ↑ "Stretchiest skin". Guinness World Records. Archived from the original on 2019-03-24. Retrieved 2019-06-22.
- ↑ "Houston hits out at 'preconceived ideas' – Coronation Street News – Soaps". Digital Spy. 2010-05-22. Archived from the original on 2013-05-09. Retrieved 2014-02-27.
- ↑ "Drag Race's Yvie Oddly On Living with Ehlers Danos Syndrome". Archived from the original on 2020-08-07. Retrieved 2020-08-07.
- ↑ Rosenberg, Peter; Rosenberg, Peter (30 September 2014). "Eric the Actor: A Eulogy". Rolling Stone. Archived from the original on 22 July 2019. Retrieved 23 July 2019.
- 1 2 Gillespie, Claire. "Jameela Jamil Confirms She Has Ehlers-Danlos Syndrome". SELF. Archived from the original on 9 August 2019. Retrieved 9 August 2019.
- ↑ ""Lena Dunham goes on Instagram to reveal she has Ehlers-Danlos syndrome"". Archived from the original on 2020-08-03. Retrieved 2020-08-07.
- ↑ Doherty, Jennifer (2019-10-05). "Ehlers-Danlos syndrome: Singer Sia's condition explained". Newsweek. Archived from the original on 2019-11-11. Retrieved 2019-11-11.
- ↑ "Here's What YouTuber Annie Elainey Wants You to Know About Being Disabled". Brit + Co. 2017-09-01. Archived from the original on 2019-11-11. Retrieved 2019-11-11.
- ↑ "Living with little-known disorder Ehlers-Danlos sparked Miss Virginia's love of science". VCU School of Pharmacy News. Archived from the original on 2020-03-01. Retrieved 2020-03-01.
- ↑ Scott DV (October 1974). "Cutaneous asthenia in a cat, resembling Ehlers-Danlos syndrome in man". Veterinary Medicine, Small Animal Clinician. 69 (10): 1256–8. doi:10.3906/vet-1203-64. PMID 4496767.
- ↑ Scott, Danny W (2008). "Congenital and hereditary skin diseases". Color Atlas of Farm Animal Dermatology. Wiley Online Library. p. 61. doi:10.1002/9780470344460. ISBN 9780470344460.
- ↑ "Ehler-Danlos Syndrome (Cutaneous asthenia, dermatosparaxis)". veterinary-practice.com. Archived from the original on 2019-06-03. Retrieved 2019-06-03.
- ↑ Halper J (2014). "Connective tissue disorders in domestic animals". Advances in Experimental Medicine and Biology. 802: 231–40. doi:10.1007/978-94-007-7893-1_14. ISBN 978-94-007-7892-4. PMID 24443030. Archived from the original on 2021-08-28. Retrieved 2020-08-07.
External links
- Ehlers–Danlos syndromes at Curlie
| Classification | |
|---|---|
| External resources |