MMR vaccine
 Measles, mumps, and rubella vaccine (live) | |
| Combination of | |
|---|---|
| Measles vaccine | Vaccine |
| Mumps vaccine | Vaccine |
| Rubella vaccine | Vaccine |
| Names | |
| Trade names | M-M-R II, Priorix, Tresivac, others |
| Other names | MPR vaccine[1] |
| Clinical data | |
| Main uses | Prevents measles, mumps, and rubella (German measles)[2] |
| Side effects | Fever, pain or redness at injection site[3] |
| Pregnancy category | |
| External links | |
| AHFS/Drugs.com | Professional Drug Facts |
| US NLM | MMR vaccine |
| MedlinePlus | a601176 |
| Legal | |
| License data | |
| Legal status | |
The MMR vaccine is a vaccine that protects against measles, mumps, and rubella (German measles).[5] The first dose is generally given to children around 9 to 15 months of age, with a second dose at 15 months to 6 years of age, with at least 4 weeks between the doses.[6][7] After two doses, 97% of people are protected against measles, 88% against mumps, and at least 97% against rubella.[6] The vaccine is also recommended in those who do not have evidence of immunity,[6] those with well-controlled HIV/AIDS,[8][9] and within 72 hours of exposure to measles among those who are incompletely immunized.[7] It is given by injection into muscle or under the skin.[10][11]
The MMR vaccine is widely used around the world, with over 500 million doses having been given in over 100 countries as of 2001.[12][13] Measles resulted in 2.6 million deaths per year before immunization became common.[13] This has decreased to 122,000 deaths per year as of 2012, mostly in low-income countries.[13] Through vaccination, as of 2018, rates of measles in North and South America are very low.[13] Rates of disease have been seen to increase in populations which go unvaccinated.[13] Between 2000 and 2016, vaccination decreased measles deaths by a further 84%.[14]
Side effects are generally mild and go away without any specific treatment.[3] These may include fever, typically 7 and 10 days following vaccination, and a rash, 2 weeks after vaccination.[5] Other side effects include pain or redness at the injection site.[3] Severe allergic reactions occur in about one in a million people.[3] Because it contains live viruses, the MMR vaccine is not recommended during pregnancy, but may be given while breastfeeding.[6] The vaccine is safe to give at the same time as other vaccines.[3] Being recently immunized does not increase the risk of passing measles, mumps, or rubella on to others.[6] Vaccination does not increase the risk of autism.[15][16][17] The MMR vaccine is a mixture of live weakened viruses of the three diseases.[6]
The MMR vaccine was developed by Maurice Hilleman.[2] It was licensed for use by Merck in 1971.[18] Stand-alone measles, mumps, and rubella vaccines had been previously licensed in 1963, 1967, and 1969 respectively.[18][19] Recommendations for a second dose were introduced in 1989.[18] The MMRV vaccine which also covers chickenpox may be used instead.[6] An MR vaccine, without coverage for mumps, is also occasionally used.[20] In the UK, one pre-filled MMR dose costs the NHS around £10 as of 2021.[10]
Medical use
In 2020, Cochrane concluded "Existing evidence on the safety and effectiveness of MMR vaccine supports current policies of mass immunisation aimed at global measles eradication and in order to reduce morbidity and mortality associated with mumps and rubella."[15]
The combined MMR vaccine induces immunity less painfully than three separate injections at the same time, and sooner and more efficiently than three injections given on different dates. Public Health England reports that providing a single combined vaccine as of 1988 rather than giving the option to have them also done separately increased uptake of the vaccine.[21]
Measles

Before the widespread use of a vaccine against measles, rates of disease were so high that infection was felt to be "as inevitable as death and taxes."[22] Reported cases of measles in the United States fell from hundreds of thousands to tens of thousands per year following introduction of the vaccine in 1963. Increasing uptake of the vaccine following outbreaks in 1971, and 1977, brought this down to thousands of cases per year in the 1980s. An outbreak of almost 30,000 cases in 1990 led to a renewed push for vaccination and the addition of a second vaccine to the recommended schedule. Fewer than 200 cases have been reported in the U.S. each year between 1997 and 2013, and the disease is no longer considered endemic there.[23][24][25]
The benefit of measles vaccination in preventing illness, disability, and death has been well documented. The first 20 years of licensed measles vaccination in the U.S. prevented an estimated 52 million cases of the disease, 17,400 cases of intellectual disability, and 5,200 deaths.[26] During 1999–2004, a strategy led by the World Health Organization and UNICEF led to improvements in measles vaccination coverage that averted an estimated 1.4 million measles deaths worldwide.[27] Between 2000 and 2013, measles vaccination resulted in a 75% decrease in deaths from the disease.[28]
Measles is common in many areas of the world. Although it was declared eliminated from the U.S. in 2000, high rates of vaccination and good communication with persons who refuse vaccination are needed to prevent outbreaks and sustain the elimination of measles in the U.S.[29] Of the 66 cases of measles reported in the U.S. in 2005, slightly over half were attributable to one unvaccinated individual who acquired measles during a visit to Romania.[30] This individual returned to a community with many unvaccinated children. The resulting outbreak infected 34 people, mostly children and virtually all unvaccinated; 9% were hospitalized, and the cost of containing the outbreak was estimated at $167,685. A major epidemic was averted due to high rates of vaccination in the surrounding communities.[29]
In 2017, an outbreak of measles occurred among the Somali-American community in Minnesota, where MMR vaccination rates had declined due to the misconception that the vaccine could cause autism. The Centers for Disease Control and Prevention recorded 65 affected children in the outbreak by April 10, 2017.[31]
Rubella
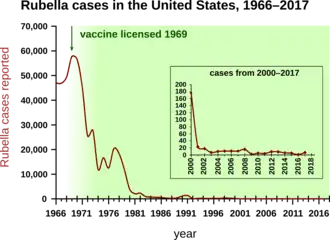
Rubella, also known as German measles, was also very common before widespread vaccination. The major risk of rubella is during pregnancy when the baby may contract congenital rubella, which can cause significant congenital defects.[32]
Mumps
Mumps is another viral disease that was once very common, especially during childhood. If mumps is acquired by a male who is past puberty, a possible complication is bilateral orchitis, which can in some cases lead to sterility.[33]
Administration
The MMR vaccine can be administered in the traditional way of injection under the skin, or by injection into muscle.[10][34] National or local guidance may vary, with some preferring one method to the other.[35] The intramuscular route has been becoming more popular.[36]
The first dose usually given at 12 months of age.[37] The second dose may be given as early as one month after the first dose.[38] The second dose is a dose to produce immunity in the small number of persons (2–5%) who fail to develop measles immunity after the first dose. In the U.S. it is done before entry to kindergarten because that is a convenient time.[39] Areas where measles is common typically recommend the first dose at 9 months of age and the second dose at 15 months of age.[7]
Safety
Adverse reactions, rarely serious, may occur from each component of the MMR vaccine. Ten percent of children develop fever, malaise, and a rash 5–21 days after the first vaccination;[40] and 3% develop joint pain lasting 18 days on average.[41] Older women appear to be more at risk of joint pain, acute arthritis, and even (rarely) chronic arthritis.[42] Anaphylaxis is an extremely rare but serious allergic reaction to the vaccine.[43] One cause can be egg allergy.[44] In 2014, the FDA approved two additional possible adverse events on the vaccination label: acute disseminated encephalomyelitis (ADEM), and transverse myelitis, with permission to also add "difficulty walking" to the package inserts.[45] A 2012 IOM report found that the measles component of the MMR vaccine can cause measles inclusion body encephalitis in immunocompromised individuals. This report also rejected any connection between the MMR vaccine and autism.[46] Some versions of the vaccine contain the antibiotic neomycin and therefore should not be used in people allergic to this antibiotic.[17]
The number of reports on neurological disorders is very small, other than evidence for an association between a form of the MMR vaccine containing the Urabe mumps strain and rare adverse events of aseptic meningitis, a form of viral meningitis.[42][47] The UK National Health Service stopped using the Urabe mumps strain in the early 1990s due to cases of transient mild viral meningitis, and switched to a form using the Jeryl Lynn mumps strain instead.[48] The Urabe strain remains in use in a number of countries; MMR with the Urabe strain is much cheaper to manufacture than with the Jeryl Lynn strain,[49] and a strain with higher efficacy along with a somewhat higher rate of mild side effects may still have the advantage of reduced incidence of overall adverse events.[48]
A Cochrane review found that, compared with placebo, MMR vaccine was associated with fewer upper respiratory tract infections, more irritability, and a similar number of other adverse effects.[15]
Naturally acquired measles often occurs with immune thrombocytopenic purpura (ITP, a purpuric rash and an increased tendency to bleed that resolves within two months in children). Approximately 1 in 40,000 children are thought to acquire ITP in the six weeks following an MMR vaccination, which is a higher rate than found in unvaccinated populations.[50] ITP below the age of six years is generally a mild disease, rarely having long-term consequences.[51][52]
False claims about autism
In 1998 Andrew Wakefield et al. published a fraudulent paper about twelve children, reportedly with bowel symptoms and autism or other disorders acquired soon after administration of MMR vaccine,[53] while supporting a competing vaccine. In 2010, Wakefield's research was found by the General Medical Council to have been "dishonest",[54] and The Lancet fully retracted the paper.[55] Three months following The Lancet's retraction, Wakefield was struck off the UK medical register, with a statement identifying deliberate falsification in the research published in The Lancet,[56] and was barred from practising medicine in the UK.[57] The research was declared fraudulent in 2011 by the British Medical Journal.[58]
Peer-reviewed studies have failed to show any association between the vaccine and autism.[15][59] The Centers for Disease Control and Prevention,[60] the Institute of Medicine of the National Academy of Sciences,[61] the UK National Health Service[62] and the Cochrane Library review[15] have all concluded that there is no evidence of a link.
Administering the vaccines in three separate doses does not reduce the chance of adverse effects, and it increases the opportunity for infection by the two diseases not immunized against first.[59][63] Health experts have criticized media reporting of the MMR-autism controversy for triggering a decline in vaccination rates.[64] Before publication of Wakefield's findings, the inoculation rate for MMR in the UK was 92%; after publication, the rate dropped to below 80%. In 1998, there were 56 measles cases in the UK; by 2008, there were 1348 cases, with two confirmed deaths.[65]
In Japan, the MMR triplet is not used. Immunity is achieved by a combination vaccine for measles and rubella, followed up later with a mumps only vaccine. This has had no effect on autism rates in the country, further disproving the MMR autism hypothesis.[66]
History


The component viral strains of MMR vaccine were developed by propagation in animal and human cells as all viruses require a living host cell to replicate.
For example, in the case of mumps and measles viruses, the virus strains were grown in embryonated chicken eggs. This produced strains of virus which were adapted for chicken cells and less well-suited for human cells. These strains are therefore called attenuated strains. They are sometimes referred to as neuroattenuated because these strains are less virulent to human neurons than the wild strains.
The Rubella component, Meruvax, was developed in 1967 through propagation using the human embryonic lung cell line WI-38 (named for the Wistar Institute) that was derived six years earlier in 1961.[67][68]
| Disease immunized | Component vaccine | Virus strain | Propagation medium | Growth medium |
|---|---|---|---|---|
| Measles | Attenuvax | Enders' attenuated Edmonston strain[69] | chick embryo cell culture | Medium 199 |
| Mumps | Mumpsvax[70] | Jeryl Lynn (B level) strain[71] | ||
| Rubella | Meruvax II | Wistar RA 27/3 strain of live attenuated rubella virus | WI-38 human embryonic cell line | MEM (solution containing buffered salts, fetal bovine serum, human serum albumin and neomycin, etc.) |
MMR II is supplied freeze-dried (lyophilized) and contains live viruses. Before injection it is reconstituted with the solvent provided.
The term "MPR vaccine" is based on the Latin names of the diseases.
The MMR vaccine Pluserix (known as Trivirix in Canada) uses the Urabe mumps strain. It is no longer used in the UK or Canada, but it remains in use in a number of countries.[72]
MMRV vaccine
The MMRV vaccine, a combined measles, mumps, rubella and varicella (chickenpox) vaccine, has been proposed as a replacement for the MMR vaccine to simplify administration of the vaccines.[38] Preliminary data indicate a rate of febrile seizures of 9 per 10,000 vaccinations with MMRV, as opposed to 4 per 10,000 for separate MMR and varicella shots; U.S. health officials therefore do not express a preference for use of MMRV vaccine over separate injections.[73]
In a 2012 study[74] pediatricians and family doctors were sent a survey to gauge their awareness of the increased risk of febrile seizures (fever fits) in the MMRV. 74% of family doctors and 29% of pediatricians were unaware of the increased risk of febrile seizures. After reading an informational statement only 7% of family doctors and 20% of pediatricians would recommend the MMRV for a healthy 12- to 15-month-old child. The factor that was reported as the "most important" deciding factor in recommending the MMRV over the MMR+V was ACIP/AAFP/AAP recommendations (pediatricians, 77%; family physicians, 73%).
MR vaccine
This is a vaccine that covers measles and rubella but not mumps.[20] It is used in a few areas of the world as of 2014.[20]
Religious concerns
Some brands of this vaccine use gelatine, derived from domestic pigs, as a stabilizer. This has caused reduced take-up, and consequent increased levels of disease, among communities with a high proportion of Muslims or Orthodox Jews.[75][76]
References
- ↑ Grignolio, Andrea (2018). Vaccines: Are they Worth a Shot?. Springer. p. 2. ISBN 9783319681061. Archived from the original on 2021-04-17. Retrieved 2020-05-22.
- 1 2 "Maurice R. Hilleman, PhD, DSc". Seminars in Pediatric Infectious Diseases. 16 (3): 225–226. July 2005. doi:10.1053/j.spid.2005.05.002. PMID 16044396.
- 1 2 3 4 5 "Vaccine Information Statement Measles-Mumps-Rubella". Centers for Disease Control and Prevention (CDC). 11 July 2018. Archived from the original on 3 September 2018. Retrieved 10 September 2018.
- 1 2 Use During Pregnancy and Breastfeeding
- 1 2 Vesikari, Timo; Usonis, Vytautas (2021). "9. Measles-Mumps-Rubella vaccine". In Vesikari, Timo; Damme, Pierre Van (eds.). Pediatric Vaccines and Vaccinations: A European Textbook (Second ed.). Switzerland: Springer. pp. 79–90. ISBN 978-3-030-77172-0. Archived from the original on 2022-01-11. Retrieved 2022-01-27.
- 1 2 3 4 5 6 7 "MMR Vaccination What You Should Know Measles, Mumps, Rubella". Centers for Disease Control and Prevention (CDC). 2 February 2018. Archived from the original on 26 April 2020. Retrieved 10 September 2018.
- 1 2 3 World Health Organization (2017). "Measles vaccines: WHO position paper – April 2017". Wkly. Epidemiol. Rec. 92 (17): 205–27. hdl:10665/255149. PMID 28459148. Lay summary (PDF).
{{cite journal}}: Cite uses deprecated parameter|lay-url=(help) - ↑ Kinney, Rebecca (2 May 2017). "Core Concepts - Immunizations in Adults - Basic HIV Primary Care - National HIV CurriculumImmunizations in Adults". www.hiv.uw.edu. Archived from the original on 2 September 2018. Retrieved 8 May 2019.
- ↑ Watson JC, Hadler SC, Dykewicz CA, Reef S, Phillips L (22 May 1998). "Measles, mumps, and rubella--vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP)" (PDF). MMWR Recomm Rep. 47 (RR-8): 1–57. PMID 9639369. Archived (PDF) from the original on 30 October 2019. Retrieved 26 January 2020.
- 1 2 3 "14. Vaccines". British National Formulary (BNF) (82 ed.). London: BMJ Group and the Pharmaceutical Press. September 2021 – March 2022. pp. 1390–1391. ISBN 978-0-85711-413-6.
{{cite book}}: CS1 maint: date format (link) - ↑ Lampiris, Harry W.; maddix, Daniel S. (2020). "Appendix: Vaccines, immune globulins, and other complex biologic products". In Katzung, Bertram G.; Trevor, Anthony J. (eds.). Basic and Clinical Pharmacology (15th ed.). New York: McGraw-Hill. p. 1229. ISBN 978-1-260-45231-0. Archived from the original on 2021-10-10. Retrieved 2021-12-28.
- ↑ "Top 10 myths about MMR, Top 10 truths about MMR" (PDF). NHS. Archived (PDF) from the original on 11 September 2018. Retrieved 10 September 2018.
- 1 2 3 4 5 "Addressing misconceptions on measles vaccination". European Centre for Disease Prevention and Control. Archived from the original on 11 September 2018. Retrieved 10 September 2018.
- ↑ "Measles Fact Sheet #286". World Health Organization (WHO). Archived from the original on 3 February 2015. Retrieved 1 December 2014.
- 1 2 3 4 5 Di Pietrantonj, C; Rivetti, A; Marchione, P; Debalini, MG; Demicheli, V (April 2020). "Vaccines for measles, mumps, rubella, and varicella in children". Cochrane Database of Systematic Reviews. 4: CD004407. doi:10.1002/14651858.CD004407.pub4. PMC 7169657. PMID 32309885.
- ↑ Hussain A, Ali S, Ahmed M, Hussain S (3 July 2018). "The Anti-vaccination Movement: A Regression in Modern Medicine". Cureus. 10 (7): e2919. doi:10.7759/cureus.2919. PMC 6122668. PMID 30186724.
- 1 2 Spencer JP, Trondsen Pawlowski RH, Thomas S (15 June 2017). "Vaccine Adverse Events: Separating Myth from Reality". American Family Physician. 95 (12): 786–794. PMID 28671426.
- 1 2 3 Goodson, JL; Seward, JF (December 2015). "Measles 50 Years After Use of Measles Vaccine". Infectious Disease Clinics of North America. 29 (4): 725–43. doi:10.1016/j.idc.2015.08.001. PMID 26610423.
- ↑ "Measles: information about the disease and vaccines Questions and Answers" (PDF). Immunization Action Coalition. Archived (PDF) from the original on 24 January 2013. Retrieved 10 September 2018.
- 1 2 3 "Information Sheet Observed Rate of Vaccine Reactions, Measles, Mumps, and Rubella Vaccines" (PDF). World Health Organization (WHO). May 2014. Archived (PDF) from the original on 2 December 2018. Retrieved 10 September 2018.
- ↑ "Measles, mumps, rubella (MMR): use of combined vaccine instead of single vaccines". GOV.UK. Archived from the original on 12 July 2018. Retrieved 12 July 2018.
- ↑ Babbott FL Jr; Gordon JE (1954). "Modern measles". Am J Med Sci. 228 (3): 334–61. doi:10.1097/00000441-195409000-00013. PMID 13197385.
- ↑ Centers for Disease Control and Prevention (CDC) (October 1994). "Summary of notifiable diseases, United States, 1993" (PDF). MMWR Morb. Mortal. Wkly. Rep. 42 (53): i–xvii, 1–73. PMID 9247368. Archived (PDF) from the original on 2020-10-24. Retrieved 2020-01-26.
- ↑ Centers for Disease Control and Prevention (CDC) (July 2009). "Summary of Notifiable Diseases --- United States, 2007" (PDF). MMWR Morb. Mortal. Wkly. Rep. 56 (53). Archived (PDF) from the original on 2020-10-24. Retrieved 2020-01-26.
- ↑ Hamborsky J, Kroger A, Wolfe S, eds. (2015). Epidemiology and Prevention of Vaccine-Preventable Diseases (13th ed.). Washington D.C.: U.S. Centers for Disease Control and Prevention (CDC). ISBN 978-0990449119. Archived from the original on 2016-12-30. Retrieved 2017-09-09.
- ↑ Bloch AB, Orenstein WA, Stetler HC, et al. (1985). "Health impact of measles vaccination in the United States". Pediatrics. 76 (4): 524–32. PMID 3931045.
- ↑ Centers for Disease Control and Prevention (CDC) (March 2006). "Progress in reducing global measles deaths, 1999–2004" (PDF). MMWR Morb. Mortal. Wkly. Rep. 55 (9): 247–9. PMID 16528234. Archived (PDF) from the original on 2021-03-05. Retrieved 2020-01-26.
- ↑ "Measles Fact Sheet #286". World Health Organization (WHO). Archived from the original on 3 February 2015. Retrieved 1 December 2014.
- 1 2 Parker AA, Staggs W, Dayan GH, Ortega-Sánchez IR, Rota PA, Lowe L, Boardman P, Teclaw R, Graves C, LeBaron CW (2006). "Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States". N Engl J Med. 355 (5): 447–55. doi:10.1056/NEJMoa060775. PMID 16885548. Archived from the original on 2021-08-28. Retrieved 2019-12-01.
- ↑ Centers for Disease Control and Prevention (CDC) (December 2006). "Measles—United States, 2005" (PDF). MMWR Morb. Mortal. Wkly. Rep. 55 (50): 1348–51. PMID 17183226. Archived (PDF) from the original on 2021-01-26. Retrieved 2020-01-26.
- ↑ Hall V, Banerjee E, Kenyon C, Strain A, Griffith J, Como-Sabetti K, Heath J, Bahta L, Martin K, McMahon M, Johnson D, Roddy M, Dunn D, Ehresmann K (July 2017). "Measles Outbreak — Minnesota April–May 2017" (PDF). MMWR Morb. Mortal. Wkly. Rep. 66 (27): 713–717. doi:10.15585/mmwr.mm6627a1. ISSN 0149-2195. PMC 5687591. PMID 28704350. Archived (PDF) from the original on 2020-08-02. Retrieved 2020-01-26.
- ↑ "Rubella vaccine information". National Network for Immunization Information. 2006-09-25. Archived from the original on 2007-09-27. Retrieved 2007-09-02.
- ↑ Jequier, Anne M. (2000). Male infertility: a guide for the clinician. Malden, MA: Blackwell Publishing. p. 118. ISBN 978-0-632-05129-8. Archived from the original on 2021-04-17. Retrieved 2016-09-24.
- ↑ Gillet, Yves; Habermehl, Pirmin; Thomas, Stéphane; Eymin, Cécile; Fiquet, Anne (14 April 2009). "Immunogenicity and safety of concomitant administration of a measles, mumps and rubella vaccine (M-M-RvaxPro®) and a varicella vaccine (VARIVAX®) by intramuscular or subcutaneous routes at separate injection sites: a randomised clinical trial". BMC Medicine. 7 (1): 16. doi:10.1186/1741-7015-7-16. ISSN 1741-7015. PMID 19366435. Archived from the original on 2021-12-20. Retrieved 2022-01-26.
- ↑ Haas, Hervé; Richard, Patrick; Eymin, Cécile; Fiquet, Anne; Kuter, Barbara; Soubeyrand, Benoit (8 January 2019). "Immunogenicity and safety of intramuscular versus subcutaneous administration of a combined measles, mumps, rubella, and varicella vaccine to children 12 to 18 months of age". Human Vaccines & Immunotherapeutics. 15 (4): 778–785. doi:10.1080/21645515.2018.1549452. ISSN 2164-5515. PMID 30481110. Archived from the original on 26 January 2021. Retrieved 26 January 2022.
- ↑ Cook, Ian F. (4 May 2021). "Subcutaneous vaccine administration – an outmoded practice". Human Vaccines & Immunotherapeutics. 17 (5): 1329–1341. doi:10.1080/21645515.2020.1814094. ISSN 2164-5515. PMID 32991241.
- ↑ "Administering MMR Vaccine | CDC". Centers for Disease Control and Prevention. 26 January 2021. Archived from the original on 28 December 2021. Retrieved 28 December 2021.
- 1 2 Vesikari T, Sadzot-Delvaux C, Rentier B, Gershon A (2007). "Increasing coverage and efficiency of measles, mumps, and rubella vaccine and introducing universal varicella vaccination in Europe: a role for the combined vaccine". Pediatr Infect Dis J. 26 (7): 632–8. doi:10.1097/INF.0b013e3180616c8f. PMID 17596807.
- ↑ "MMR vaccine questions and answers". Centers for Disease Control and Prevention (CDC). 2004. Archived from the original on July 25, 2008. Retrieved 2008-05-28.
- ↑ Harnden A, Shakespeare J (2001). "10-minute consultation: MMR immunisation". BMJ. 323 (7303): 32. doi:10.1136/bmj.323.7303.32. PMC 1120664. PMID 11440943. Archived from the original on 2007-10-16. Retrieved 2007-09-25.
- ↑ Thompson GR, Ferreyra A, Brackett RG (1971). "Acute Arthritis Complicating Rubella Vaccination" (PDF). Arthritis & Rheumatism. 14 (1): 19–26. doi:10.1002/art.1780140104. hdl:2027.42/37715. PMID 5100638. Archived (PDF) from the original on 2011-11-25. Retrieved 2019-09-01.
- 1 2 Schattner A (2005). "Consequence or coincidence? The occurrence, pathogenesis and significance of autoimmune manifestations after viral vaccines". Vaccine. 23 (30): 3876–86. doi:10.1016/j.vaccine.2005.03.005. PMID 15917108.
- ↑ Carapetis JR, Curtis N, Royle J (2001). "MMR immunisation. True anaphylaxis to MMR vaccine is extremely rare". BMJ. 323 (7317): 869. doi:10.1136/bmj.323.7317.869a. PMC 1121404. PMID 11683165.
- ↑ Fox A, Lack G (October 2003). "Egg allergy and MMR vaccination". Br J Gen Pract. 53 (495): 801–2. PMC 1314715. PMID 14601358. Archived from the original on 2013-01-26.
- ↑ "Approval for label change". Archived from the original on 2017-07-23. Retrieved 2019-12-16.
- ↑ Medicine, Institute of; Practice, Board on Population Health Public Health; Vaccines, Committee to Review Adverse Effects of; Ford, A; Rusch, E; Clayton, E. W (26 March 2012). Adverse Effects of Vaccines. nap.edu. doi:10.17226/13164. ISBN 978-0-309-21435-3. PMID 24624471. Archived from the original on 7 November 2017. Retrieved 17 August 2017.
- ↑ Institute of Medicine (1994). "Measles and mumps vaccines". Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality. National Academy Press. ISBN 978-0-309-07496-4.
{{cite book}}:|access-date=requires|url=(help);|archive-url=requires|url=(help); Unknown parameter|chapterurl=ignored (help) - 1 2 Colville A, Pugh S, Miller E, Schmitt HJ, Just M, Neiss A (1994). "Withdrawal of a mumps vaccine". Eur J Pediatr. 153 (6): 467–8. doi:10.1007/BF01983415. PMID 8088305.
- ↑ Fullerton KE, Reef SE (2002). "Commentary: Ongoing debate over the safety of the different mumps vaccine strains impacts mumps disease control". Int J Epidemiol. 31 (5): 983–4. doi:10.1093/ije/31.5.983. PMID 12435772. Archived from the original on 2008-08-28. Retrieved 2007-09-25.
- ↑ Marshall, GS (2013). "Roots of Vaccine Hesitancy". S D Med (Review). Spec no: 52–7. PMID 23444592.
- ↑ Sauvé LJ, Scheifele D (January 2009). "Do childhood vaccines cause thrombocytopenia?". Paediatr Child Health. 14 (1): 31–2. doi:10.1093/pch/14.1.31. PMC 2661332. PMID 19436461.
- ↑ Black, C., Kaye, J. A. and Jick, H. (2003). "MMR vaccine and idiopathic thrombocytopaenic purpura". British Journal of Clinical Pharmacology. 55 (1): 107–111. doi:10.1046/j.1365-2125.2003.01790.x. PMC 1884189. PMID 12534647.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Wakefield A, Murch S, Anthony A; et al. (1998). "Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children". Lancet. 351 (9103): 637–41. doi:10.1016/S0140-6736(97)11096-0. PMID 9500320. Archived from the original on 2007-09-27. Retrieved 2007-09-05.
{{cite journal}}: CS1 maint: multiple names: authors list (link) (Retracted) - ↑ Cassandra Jardine (29 Jan 2010). "GMC brands Dr Andrew Wakefield 'dishonest, irresponsible and callous'". The Telegraph. London: Telegraph Media Group, Ltd. Archived from the original on 22 June 2018. Retrieved 31 January 2015.
- ↑ The Editors Of The Lancet (February 2010). "Retraction—Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children". Lancet. 375 (9713): 445. doi:10.1016/S0140-6736(10)60175-4. PMID 20137807.
{{cite journal}}:|last1=has generic name (help) - ↑ "General Medical Council, Fitness to Practise Panel Hearing, 24 May 2010, Andrew Wakefield, Determination of Serious Professional Misconduct" (PDF). General Medical Council. Archived from the original (PDF) on 9 August 2011. Retrieved 18 September 2011.
- ↑ Meikle, James; Boseley, Sarah (24 May 2010). "MMR row doctor Andrew Wakefield struck off register". The Guardian. London. Archived from the original on 27 May 2010. Retrieved 24 May 2010.
- ↑ Godlee F, Smith J, Marcovitch H (2011). "Wakefield's article linking MMR vaccine and autism was fraudulent". BMJ. 342 (jan05 1, c7452): c7452. doi:10.1136/bmj.c7452. PMID 21209060. Archived from the original on 2021-08-28. Retrieved 2019-12-01.
- 1 2 National Health Service (2004). "MMR: myths and truths". Archived from the original on 2008-09-13. Retrieved 2007-09-03.
- ↑ "Measles, mumps, and rubella (MMR) vaccine". Centers for Disease Control and Prevention. 2008-08-22. Archived from the original on 2008-10-08. Retrieved 2008-12-21.
- ↑ Immunization Safety Review: Vaccines and Autism Archived 2007-06-23 at the Wayback Machine. From the Institute of Medicine of the National Academy of Sciences. Report dated May 17, 2004; accessed June 13, 2007.
- ↑ MMR Fact Sheet Archived 2007-06-15 at the Wayback Machine, from the United Kingdom National Health Service. Accessed June 13, 2007.
- ↑ MMR vs three separate vaccines:
- Halsey NA; Hyman SL; Conference Writing Panel (2001). "Measles-mumps-rubella vaccine and autistic spectrum disorder: report from the New Challenges in Childhood Immunizations Conference convened in Oak Brook, Illinois, June 12–13, 2000". Pediatrics. 107 (5): e84. doi:10.1542/peds.107.5.e84. PMID 11331734. Archived from the original on 2009-04-05. Retrieved 2007-11-26.
- Leitch R, Halsey N, Hyman SL (2002). "MMR—Separate administration-has it been done?". Pediatrics. 109 (1): 172. doi:10.1542/peds.109.1.172. PMID 11773568. Archived from the original on 2007-02-16. Retrieved 2007-11-26.
- Miller E (2002). "MMR vaccine: review of benefits and risks". J Infect. 44 (1): 1–6. doi:10.1053/jinf.2001.0930. PMID 11972410.
- ↑ "Doctors issue plea over MMR jab". BBC News. 2006-06-26. Archived from the original on 2018-07-07. Retrieved 2009-02-04.
- ↑ Thomas J (2010). "Paranoia strikes deep: MMR vaccine and autism". Psychiatric Times. 27 (3): 1–6. Archived from the original on 2015-04-09.
- ↑ Honda H, Shimizu Y, Rutter M (2005). "No effect of MMR withdrawal on the incidence of autism: a total population study". J Child Psychol Psychiatry. 46 (6): 572–9. CiteSeerX 10.1.1.579.1619. doi:10.1111/j.1469-7610.2005.01425.x. PMID 15877763.
- ↑ Plotkin SA, Vaheri A (1967). "Human fibroblasts infected with rubella virus produce a growth inhibitor". Science. 156 (3775): 659–61. Bibcode:1967Sci...156..659P. doi:10.1126/science.156.3775.659. PMID 6023662.
- ↑ Hayflick L, Moorhead PS (1967). "The serial cultivation of human diploid cell strains". Exp. Cell Res. 25 (3): 585–621. doi:10.1016/0014-4827(61)90192-6. PMID 13905658.
- ↑ "Attenuvax Product Sheet" (PDF). Merck & Co. 2006. p. 1. Archived from the original (PDF) on 2009-12-31. Retrieved 2009-02-04.
- ↑ Merck Co. (2002). "MUMPSVAX (Mumps Virus Vaccine Live) Jeryl Lynn Strain" (PDF). Merck Co. Archived from the original (PDF) on 2006-08-13. Retrieved 2015-01-26.
- ↑ Young ML, Dickstein B, Weibel RE, Stokes J Jr, Buynak EB, Hilleman MR (1967). "Experiences with Jeryl Lynn strain live attenuated mumps virus vaccine in a pediatric outpatient clinic". Pediatrics. 40 (5): 798–803. PMID 6075651.
- ↑ "A look back at the so called "CDC Whistleblower" story and how Vaxxed is misleading". 2017-02-10. Archived from the original on 2019-01-12. Retrieved 2019-05-08.
- ↑ Klein NP, Yih WK, Marin M, et al. (March 2008). "Update: recommendations from the Advisory Committee on Immunization Practices (ACIP) regarding administration of combination MMRV vaccine" (PDF). MMWR Morb. Mortal. Wkly. Rep. 57 (10): 258–60. PMID 18340332. Archived (PDF) from the original on 2020-10-19. Retrieved 2020-01-26.
- ↑ O'Leary ST, Suh CA, Marin M (Nov 2012). "Febrile seizures and measles-mumps-rubella-varicella (MMRV) vaccine: what do primary care physicians think?". Vaccine. 30 (48): 6731–3. doi:10.1016/j.vaccine.2012.08.075. PMID 22975026.
- ↑ Pager, Tyler (9 April 2019). "'Monkey, Rat and Pig DNA': How Misinformation Is Driving the Measles Outbreak Among Ultra-Orthodox Jews". The New York Times. Archived from the original on 14 April 2019. Retrieved 14 April 2019.
- ↑ "Vaccines and porcine gelatine" (PDF). Public Health England. August 2015. Archived (PDF) from the original on 14 April 2019. Retrieved 14 April 2019.
Further reading
- World Health Organization (January 2009). The immunological basis for immunization series: module 7: measles (update 2009). World Health Organization (WHO). hdl:10665/44038. ISBN 9789241597555.
- World Health Organization (November 2010). The immunological basis for immunization series: module 16: mumps. World Health Organization (WHO). hdl:10665/97885. ISBN 9789241500661.
- World Health Organization (December 2008). The immunological basis for immunization series: module 11: rubella. World Health Organization (WHO). hdl:10665/43922. ISBN 9789241596848.
- Ramsay, Mary, ed. (December 2019). "Chapter 21: Measles". Immunisation against infectious disease. Public Health England. Archived from the original on 2019-11-12. Retrieved 2020-01-26.
- Ramsay, Mary, ed. (April 2013). "Chapter 23: Mumps". Immunisation against infectious disease. Public Health England. Archived from the original on 2019-11-12. Retrieved 2020-01-26.
- Ramsay, Mary, ed. (April 2013). "Chapter 28: Rubella". Immunisation against infectious disease. Public Health England. Archived from the original on 2019-11-12. Retrieved 2020-01-26.
- Hamborsky J, Kroger A, Wolfe S, eds. (2015). "Chapter 13: Measles". Epidemiology and Prevention of Vaccine-Preventable Diseases (13th ed.). Washington D.C.: U.S. Centers for Disease Control and Prevention (CDC). ISBN 978-0990449119. Archived from the original on 2016-12-30. Retrieved 2017-09-09.
- Hamborsky J, Kroger A, Wolfe S, eds. (2015). "Chapter 15: Mumps". Epidemiology and Prevention of Vaccine-Preventable Diseases (13th ed.). Washington D.C.: U.S. Centers for Disease Control and Prevention (CDC). ISBN 978-0990449119. Archived from the original on 2016-12-30. Retrieved 2017-09-09.
- Hamborsky J, Kroger A, Wolfe S, eds. (2015). "Chapter 20: Rubella". Epidemiology and Prevention of Vaccine-Preventable Diseases (13th ed.). Washington D.C.: U.S. Centers for Disease Control and Prevention (CDC). ISBN 978-0990449119. Archived from the original on 2016-12-30. Retrieved 2017-09-09.
- Roush, Sandra W.; Baldy, Linda M.; Hall, Mary Ann Kirkconnell, eds. (March 2019). "Chapter 7: Measles". Manual for the surveillance of vaccine-preventable diseases. Atlanta GA: U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on 2020-08-01. Retrieved 2020-01-26.
- Roush, Sandra W.; Baldy, Linda M.; Hall, Mary Ann Kirkconnell, eds. (March 2019). "Chapter 9: Mumps". Manual for the surveillance of vaccine-preventable diseases. Atlanta GA: U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on 2020-08-01. Retrieved 2020-01-26.
- Roush, Sandra W.; Baldy, Linda M.; Hall, Mary Ann Kirkconnell, eds. (March 2019). "Chapter 14: Rubella". Manual for the surveillance of vaccine-preventable diseases. Atlanta GA: U.S. Centers for Disease Control and Prevention (CDC). Archived from the original on 2020-08-01. Retrieved 2020-01-26.
External links
| Identifiers: |
|---|
- "MMR (Measles, Mumps, & Rubella) Vaccine Information Statement". Centers for Disease Control and Prevention (CDC). Archived from the original on 2018-09-03. Retrieved 2017-09-09.
- Measles-Mumps-Rubella Vaccine at the US National Library of Medicine Medical Subject Headings (MeSH)
- "Measles Mumps Rubella Vaccines". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-04-17. Retrieved 2020-05-16.