γ-Cyclodextrin
γ-Cyclodextrin sometimes abbreviated as γ-CD, is an octasaccharide derived from glucose. The α- (alpha), β- (beta), and γ- (gamma) cyclodextrins correspond to six, seven, and eight glucose units, respectively.
 | |
| Names | |
|---|---|
| IUPAC name
cyclomaltooctaaose | |
| Systematic IUPAC name
cyclooctaakis-(1→4)-α-D-glucopyranosyl | |
| Other names
Cyclooctaamylose Cyclooctadextrin gammadex ringdex C | |
| Identifiers | |
| |
3D model (JSmol) |
|
| 78740 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.037.696 |
| E number | E458 (thickeners, ...) |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C48H80O40 | |
| Molar mass | 1297.128 g·mol−1 |
| Appearance | white solid |
| Density | 1.41 g/cm3 |
| Melting point | 474 °C (885 °F; 747 K) at fast heating rates, decomposition below 300 °C for conventional heating [1] |
| 23.2 g/100 mL | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Structure
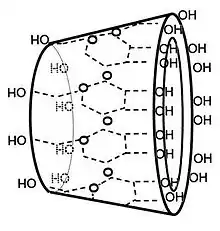
In γ-cyclodextrin, the eight glucose subunits are linked end to end via α-1,4 linkages. The result has the shape of a tapered cylinder, with 8 primary alcohols on one face and 16 secondary alcohol groups on the other. The exterior surface of cyclodextrins is somewhat hydrophilic whereas the interior core is hydrophobic.
Physical properties
γ-Cyclodextrin exists as a white (colorless) powder or crystals. The density of its hydrate crystal (γCD·14H2O) is 1.41 g/cm3. γ-Cyclodextrin is well soluble in water and dimethyl sulfoxide, poorly soluble in methanol.[2]
Applications
γ-Cyclodextrins has the largest cavity size between natural cyclodextrin, thus, it is well-suited to accommodate larger biomolecules and other guests. For this reason, γ-cyclodextrin is most commonly used as a complexing agent. γ-Cyclodextrin is widely used in medicine, pharmacy, food industry, cosmetics, textiles.
Derivatives
To increase solubility, hydroxypropylated γ-cyclodextrin derivative (HPγCD) is obtained by treating the natural cyclodextrin with propylene oxide, and sulfobutylether γ-cyclodextrin (SBEγCD) by treating the natural CD with 1,4-butane sultone.[3] Sugammadex is the derivative of γ-cyclodextrin applied as a medication.
References
- Gatiatulin, Askar (2022), "Determination of Melting Parameters of Cyclodextrins Using Fast Scanning Calorimetry", Int. J. Mol. Sci., 23 (21): 13120, doi:10.3390/ijms232113120, PMC 9655725, PMID 36361919
- Gamma-cyclodextrin
- Jansook, Phatsawee (2018), "Cyclodextrins: structure, physicochemical properties and pharmaceutical applications", Int. J. Pharm., 535 (1–2): 272–284, doi:10.1016/j.ijpharm.2017.11.018, PMID 29138045