1,1-Dichloro-1-fluoroethane
1,1-Dichloro-1-fluoroethane is a haloalkane with the formula C
2H
3Cl
2F. It is one of the three isomers of dichlorofluoroethane. It belongs to the hydrochlorofluorocarbon (HCFC) family of man-made compounds that contribute significantly to both ozone depletion and global warming when released into the environment.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,1-Dichloro-1-fluoroethane | |||
| Other names
Dichlorofluoroethane; R-141b; HCFC-141b | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.100.575 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 9274 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H3Cl2F | |||
| Molar mass | 116.94 g·mol−1 | ||
| Appearance | Colorless liquid, ethereal odor | ||
| Density | 1.25 g/cm3 at 20 °C[1] | ||
| Melting point | −103.5 °C (−154.3 °F; 169.7 K)[1] | ||
| Boiling point | 32 °C (90 °F; 305 K)[1] | ||
| 4 g/L (20 °C)[1] | |||
| Hazards | |||
| GHS labelling: | |||
 | |||
| Warning | |||
| H412, H420 | |||
| P273, P501, P502 | |||
| 532 °C (990 °F; 805 K)[1] | |||
| Explosive limits | 5.6–17.7% vol.[1] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
5 g/kg (rat, oral) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Physiochemical properties
1,1-Dichloro-1-fluoroethane can be a non-flammable, colourless liquid under room-temperature atmospheric conditions. The compound is very volatile with a boiling point of 32°C.[1][2] Its critical temperature is near 204°C.[3] Its smell has been described as "usually ethereal" (like ether).
Production and use
1,1-Dichloro-1-fluoroethane is mainly used as a solvent and foam blowing agent under the names R-141b and HCFC-141b. It is a class 2 ozone depleting substance undergoing a global phaseout from production and use under the Montreal Protocol since the late 1990s. It is being replaced by HFCs within some applications.[4]
Environmental effects
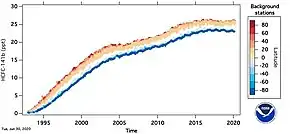
The concentration of HCFC-141b in the atmosphere grew to near 25 parts per trillion by year 2016.[5] It has an ozone depletion potential (ODP) of 0.12.[6] This is low compared to the ODP=1 of trichlorofluoromethane (CFC-11, R-11), which also grew about ten times more abundant in the atmosphere prior to introduction of HFC-141b and subsequent adoption of the Montreal Protocol.
HFC-141b is also a minor but potent greenhouse gas. It has an estimated lifetime of about 10 years and a 100-year global warming potential ranging 725 to 2500.[7][8] This compares to the GWP=1 of carbon dioxide, which had a much greater atmospheric concentration near 400 parts per million in year 2020.
References
- Record of 1,1-Dichloro-1-fluoroethane in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 8 February 2009.
- "Addenda d, j, l, m, and t to ANSI/ASHRAE Standard 34-2004" (PDF). ANSI/ASHRAE Standard 34-2004, Designation and Safety Classification of Refrigerants. Atlanta, GA: American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc. 2007-03-03. ISSN 1041-2336. Archived from the original (PDF) on 2011-10-12. Retrieved 2011-12-18.
- Schoen, J. Andrew, "Listing of Refrigerants" (PDF), Andy's HVAC/R Web Page, archived from the original (PDF) on 2009-03-19, retrieved 2011-12-17
- "Overview of HCFC Consumption and Available Alternatives For Article 5 Countries" (PDF). ICF International. 2008. Retrieved 2021-02-12.
- "HCFC-141b". NOAA Earth System Research Laboratories/Global Monitoring Division. Retrieved 2021-02-12.
- John S. Daniel; Guus J.M. Velders; A.R. Douglass; P.M.D. Forster; D.A. Hauglustaine; I.S.A. Isaksen; L.J.M. Kuijpers; A. McCulloch; T.J. Wallington (2006). "Chapter 8. Halocarbon Scenarios, Ozone Depletion Potentials, and Global Warming Potentials" (PDF). Scientific Assessment of Ozone Depletion: 2006. Geneva, Switzerland: World Meteorological Organization. Retrieved 9 October 2016.
- "Chapter 8". AR5 Climate Change 2013: The Physical Science Basis. p. 731.
- "Refrigerants - Environmental Properties". The Engineering ToolBox. Retrieved 2016-09-12.