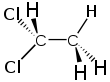
1,1-Dichloroethane
1,1-Dichloroethane is a chlorinated hydrocarbon. It is a colorless oily liquid with a chloroform-like odor. It is not easily soluble in water, but miscible with most organic solvents.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,1-Dichloroethane | |||
| Other names
Ethylidene dichloride Ethylidene chloride CFC-150a 1,1-DCA Asymmetrical dichloroethane 1,1-Ethylidene dichloride Asymmetric dichloroethane | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.785 | ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H4Cl2 | |||
| Molar mass | 98.96 g/mol | ||
| Appearance | colorless, oily liquid[1] | ||
| Odor | chloroform-like[1] | ||
| Density | 1.2 g/cm3 | ||
| Melting point | −97 °C (−143 °F; 176 K) | ||
| Boiling point | 57.2 °C (135.0 °F; 330.3 K) | ||
| 0.6%[1] | |||
| Vapor pressure | 182 mmHg (20°C)[1] | ||
| −57.4·10−6 cm3/mol | |||
| Hazards | |||
| Flash point | −17 °C; 2 °F; 256 K[1] | ||
| Explosive limits | 5.4–11.4%[1] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 100 ppm (400 mg/m3)[1] | ||
REL (Recommended) |
TWA 100 ppm (400 mg/m3)[1] | ||
IDLH (Immediate danger) |
3000 ppm[1] | ||
| Related compounds | |||
Related compounds |
1,2-Dichloroethane (ethylene dichloride); *1,1-Dichloroethene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Large volumes of 1,1-dichloroethane are manufactured, with annual production exceeding 1 million pounds in the United States. It is mainly used as a feedstock in chemical synthesis, chiefly of 1,1,1-trichloroethane. It is also used as a solvent for plastics, oils and fats, as a degreaser, as a fumigant in insecticide sprays, in halon fire extinguishers, and in cementing of rubber. It is used in manufacturing of high-vacuum resistant rubber and for extraction of temperature-sensitive substances. Thermal cracking at 400–500 °C and 10 MPa yields vinyl chloride. In the past, 1,1-dichloroethane was used as a surgical inhalational anesthetic.
Safety
1,1-dichloroethane has been on the California Proposition 65 list of known carcinogens since 1990.[2]
In the atmosphere, 1,1-dichloroethane decomposes with half-life of 62 days, chiefly by reaction of photolytically produced hydroxyl radicals.
References
- NIOSH Pocket Guide to Chemical Hazards. "#0194". National Institute for Occupational Safety and Health (NIOSH).
- "1,1-Dichloroethane". California Office of Environmental Health Hazard Assessment. Retrieved 28 February 2021.
External links
- Dichloroethane and Dichloroethene on members.optushome.com.au
- ATSDR - Toxic Substances Portal
- CDC - NIOSH Pocket Guide to Chemical Hazards