1,3-dipole
In organic chemistry, a 1,3-dipolar compound or 1,3-dipole is a dipolar compound with delocalized electrons and a separation of charge over three atoms. They are reactants in 1,3-dipolar cycloadditions.[1][2]
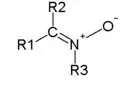 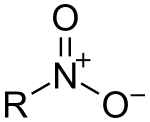 |
| From top to bottom, azides, nitrones, and nitro compounds are examples of 1,3-dipoles. |
The dipole has at least one resonance structure with positive and negative charges having a 1,3 relationship which can generally be denoted as +a−b−c−, where a may be a carbon, oxygen or nitrogen, b may be nitrogen or oxygen, and c may be a carbon, oxygen or nitrogen.[3]
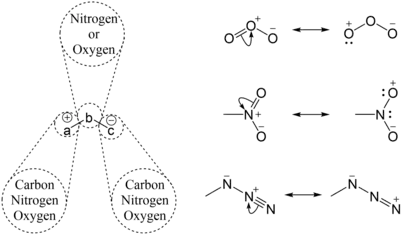
A demonstration that how some well known 1,3-dipoles like ozone, nitro compounds and azides can be shown to have a resonance structure having 1,3 relationship between positive and negative formal charges
Known 1,3-dipoles are:
- Azides (RN3)
- Ozone (O3)
- Nitro compounds (RNO2)
- Diazo compounds (R2CN2)
- Some oxides
- Azoxide compounds (RN(O)NR)
- Carbonyl oxides (Criegee zwitterions)[4][5]
- Nitrile oxides (RCN−O)
- Nitrous oxide (N2O)
- Nitrones (R2CN(R)O)
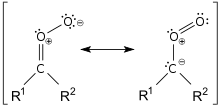
Carbonyl oxide
- Some imines:
- Azomethine imine
- Nitrilimines (RCN−NR, analogous to nitrile oxide)
- Carbonyl imines
- Some ylides
- Azomethine ylide
- Nitrile ylide (RCNCR'2)
- Carbonyl ylide
- Thiosulfines (R2CSS)
References
- Francis A. Carey, Richard J. Sundberg (2007). "Part A: Structure and mechanisms". Advanced Organic Chemistry (5, illustrated ed.). Springer. p. 874. ISBN 978-0-387-44897-8.
- IUPAC Gold Book dipolar compounds
- Jagadamba, Singh (2009). Photochemistry and Pericyclic Reactions. New Academic Science. p. 100. ISBN 978-1906574161.
- https://www.organic-chemistry.org/namedreactions/ozonolysis-criegee-mechanism.shtm Ozonolysis mechanism on Organic Chemistry Portal site
- Li, Jie Jack: Criegee mechanism of ozonolysis Book: Name Reactions. 2006, 173-174, doi:10.1007/3-540-30031-7_77
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.