Dithiane
A dithiane is a heterocyclic compound composed of a cyclohexane core structure wherein two methylene bridges (-CH
2- units) are replaced by sulfur centres. The three isomeric parent heterocycles are 1,2-dithiane, 1,3-dithiane and 1,4-dithiane.


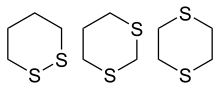 1,2-dithiane (left), 1,3-dithiane and 1,4-dithiane (right) | |
| Names | |
|---|---|
| Other names
Dithiacyclohexanes | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C4H8S2 | |
| Molar mass | 120.23 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
1,3-Dithianes
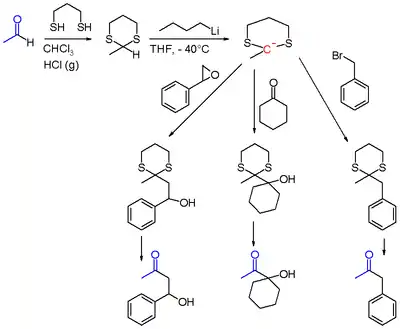
1,3-Dithianes are protecting group of some carbonyl-containing compounds due to their inertness to many conditions. They form by treatment of the carbonyl compound with 1,3-propanedithiol under conditions that remove water from the system.[1] The protecting group can be removed with mercuric reagents, a process that exploits the high affinity of Hg(II) for thiolates. 1,3-Dithianes are also employed in umpolung reactions, such as the Corey–Seebach reaction:[2]
Typically, in organic synthesis, ketones and aldehydes are protected as their dioxolanes instead of dithianes.
References
- E. J. Corey, D. Seebach (1988). "1,3-Dithiane". Organic Syntheses.; Collective Volume, vol. 6, p. 556
- T. W. Green, P. G. M. Wuts, "Protective Groups in Organic Synthesis" Wiley-Interscience, New York, 1999. ISBN 978-0-471-69754-1.