1,4-Bis(diphenylphosphino)butane
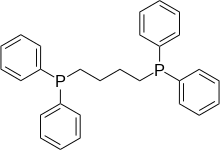
1,4-Bis(diphenylphosphino)butane (dppb) is an organophosphorus compound with the formula (Ph2PCH2CH2)2. It is less commonly used in coordination chemistry than other diphosphine ligands such as dppe. It is a white solid that is soluble in organic solvents.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(Butane-1,4-diyl)bis(diphenylphosphane) | |
| Other names
dppb, dpb | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.028.817 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C28H28P2 | |
| Molar mass | 426.480 g·mol−1 |
| Melting point | 132–136 °C (270–277 °F; 405–409 K) |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H315, H319, H335 | |
| P261, P264, P270, P271, P272, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362, P363, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Coordination complexes
Nickel complexes in which the ligand is bidentate[1][2] or monodentate[3] are known. Palladium complexes containing dppb are used in a variety of catalytic reactions.[4][5] The ligand's natural bite angle is 94° in its bidentate coordination mode.[6]
Related compounds
References
- Neary, Michelle C.; Quinlivan, Patrick J.; Parkin, Gerard (15 December 2017). "Zerovalent Nickel Compounds Supported by 1,2-Bis(diphenylphosphino)benzene: Synthesis, Structures, and Catalytic Properties". Inorganic Chemistry. 57 (1): 374–391. doi:10.1021/acs.inorgchem.7b02636. PMID 29244503.
- Nwokogu, Godson C.; Cytarska, Joanna; Zaidlewicz, Marek (15 October 2005). "Dichloro[1,4-bis(diphenylphosphino)butane]nickel(II)". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons, Ltd. doi:10.1002/047084289x.rd095.pub2. ISBN 0471936235.
- Corain, B.; Bressan, M.; Favero, G. (February 1971). "The reactions of Ni(O) phosphino complexes with carbon monoxide". Inorganic and Nuclear Chemistry Letters. 7 (2): 197–201. doi:10.1016/0020-1650(71)80151-4.
- Nozaki, Kyoko; Sato, Naomasa; Takaya, Hidemasa (May 1994). "Acylcyanation of Terminal Acetylenes: Palladium-Catalyzed Addition of Aryloyl Cyanides to Arylacetylenes". The Journal of Organic Chemistry. 59 (10): 2679–2681. doi:10.1021/jo00089a006.
- Minato, Akio; Suzuki, Keizo; Tamao, Kohei; Kumada, Makoto (1984). "Mixed heteroarene oligomers". Journal of the Chemical Society, Chemical Communications (8): 511. doi:10.1039/C39840000511.
- Birkholz (née Gensow), Mandy-Nicole; Freixa, Zoraida; van Leeuwen, Piet W. N. M. (2009). "Bite angle effects of diphosphines in C–C and C–X bond forming cross coupling reactions". Chemical Society Reviews. 38 (4): 1099. doi:10.1039/B806211K. PMID 19421583.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.