1,4-Cyclohexanedicarboxylic acid
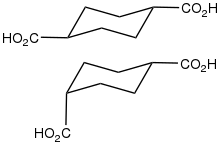
1,4-Cyclohexanedicarboxylic acid describes a pair of organic compounds with the formula C6H10(CO2H)2. The CO2H groups are attached to the opposite carbon centers of the cyclohexane ring. These groups can be cis or trans. Other isomers of cyclohexanedicarboxylic acid are known, but the 1,4- isomers are of greatest interest, perhaps because they are obtainable from a commodity chemical. Specifically, hydrogenation of terephthalic acid affords the title compound:[1]
- C6H4(CO2H)2 + 3 H2 → C6H10(CO2H)2.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.012.790 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H12O4 | |
| Molar mass | 172.180 g·mol−1 |
| Appearance | white solid |
| Density | 1.36 g/cm3 |
| Melting point | 312.5 °C (594.5 °F; 585.6 K) 168-170°C for cis isomer |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The trans isomer has been more heavily studied. It has been examined as a precursor to polycarbonates[2] and as a building block for metal-organic frameworks.[3]
References
- Maegawa, Tomohiro; Akashi, Akira; Yaguchi, Kiichiro; Iwasaki, Yohei; Shigetsura, Masahiro; Monguchi, Yasunari; Sajiki, Hironao (2009). "Efficient and Practical Arene Hydrogenation by Heterogeneous Catalysts under Mild Conditions". Chemistry - A European Journal. 15 (28): 6953–6963. doi:10.1002/chem.200900361. PMID 19514037.
- Liu, Jianwei; Yee, Albert F. (1998). "Enhancing Plastic Yielding in Polyestercarbonate Glasses by 1,4-Cyclohexylene Linkage Addition". Macromolecules. 31 (22): 7865–7870. Bibcode:1998MaMol..31.7865L. doi:10.1021/ma980370w.
- Xiang, Sheng-Chang; Zhang, Zhangjing; Zhao, Cong-Gui; Hong, Kunlun; Zhao, Xuebo; Ding, De-Rong; Xie, Ming-Hua; Wu, Chuan-De; Das, Madhab C.; Gill, Rachel; Thomas, K. Mark; Chen, Banglin (2011). "Rationally tuned micropores within enantiopure metal-organic frameworks for highly selective separation of acetylene and ethylene". Nature Communications. 2: 204. Bibcode:2011NatCo...2..204X. doi:10.1038/ncomms1206. PMID 21343922.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.