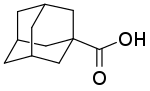
1-Adamantanecarboxylic acid
1-Adamantanecarboxylic acid is an organic compound with the formula (CH2)6(CH)3(CCO2H). A white solid, it is the simplest carboxylic acid derivative of adamantane. The compound is notable for its synthesis by carboxylation of adamantane.[1] 1-Adamantanecarboxylic acid is unusual in forming mononuclear tris(carboxylate) coordination complexes of the formula [M(O2CR)3]− (M = Mn, Ni, Co, Zn).[2]
 | |
| Names | |
|---|---|
| Other names
1-Adamantane carboxylic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.011.440 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H16O2 | |
| Molar mass | 180.247 g·mol−1 |
| Appearance | white solid |
| Melting point | 175–176.5 °C (347.0–349.7 °F; 448.1–449.6 K) |
| Related compounds | |
Related compounds |
2-Adamantanecarboxylic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- H. Koch and W. Haaf (1964). "1-Adamantanecarboxylic Acid". Organic Syntheses. 44: 1. doi:10.15227/orgsyn.044.0001.
- Fursova, E. Yu.; Romanenko, G. V.; Tolstikov, S. E.; Ovcharenko, V. I. (2019). "Mononuclear Transition Metal Adamantane-1-Carboxylates". Russian Chemical Bulletin. 68 (9): 1669–1674. doi:10.1007/s11172-019-2610-4. S2CID 203592748.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.