2,2'-Bithiophene
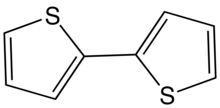
2,2′-Bithiophene is the organic compound. It is a colorless solid, although commercial samples are often greenish.[3] It is the most common of the three isomers with formula (C4H3S)2. The other two isomers have the connectivity 2,3′- and 3,3′-. The compound is typically prepared by cross-coupling starting from 2-halothiophenes.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,2′-Bithiophene | |
| Other names
2,2′-Bisthiophene, 2,2′-dithienyl, 2,2′-bithienyl, 2-(2-thienyl)thiophene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.062 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H6S2 | |
| Molar mass | 166.26 g·mol−1 |
| Appearance | Colorless crystals |
| Density | 1.44 g/cm3[1] |
| Melting point | 31.1 °C (88.0 °F; 304.2 K)[2] |
| Boiling point | 260 °C (500 °F; 533 K)[2] |
| Structure[1] | |
| Monoclinic | |
| P21/c | |
a = 7.873 A, b = 5.771 A, c = 8.813 A α = 90°, β = 107.07°, γ = 90° | |
| 2 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
X-ray crystallography shows that the two rings are coplanar,[3] unlike the situation for biphenyl.
Occurrence
A number of bi- as well as terthiophenes exist naturally, invariably with substituents at the positions flanking sulfur. In terms of the biosynthesis, bithiophenes are proposed to be derived from polyacetylenic precursors, which in turn are the products of dehydrogenation of oleic acid. According to some hypotheses, these polyalkynes form labile 1,2-dithiins via a reaction with H2S2 or its equivalent.[4]
References
- Pelletier, M.; Brisse, F. (1994). "Bithiophene at 133 K". Acta Crystallographica Section C Crystal Structure Communications. 50 (12): 1942–1945. doi:10.1107/S010827019301011X.
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 3.58. ISBN 9781498754293.
- Chaloner, P. A.; Gunatunga, S. R.; Hitchcock, P. B. (1994). "Redetermination of 2,2′-bithiophene". Acta Crystallographica C. C50 (12): 1941–2. doi:10.1107/S0108270194001149.
- Kagan, J. (1991). "Naturally occurring di- and trithiophenes". Progress in the Chemistry of Organic Natural Products. Fortschritte der Chemie organischer Naturstoffe / Progress in the Chemistry of Organic Natural Products. 56: 87–169. doi:10.1007/978-3-7091-9084-5_2. ISBN 978-3-7091-9086-9. PMID 2050313.