2,6-Dimethylnaphthalene
2,6-Dimethylnaphthalene (2,6-DMN) is a polycyclic aromatic hydrocarbon. It is one of the ten dimethylnaphthalene isomers, which are derived from naphthalene by the addition of two methyl groups.[1]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,6-Dimethylnaphthalene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.605 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H12 | |
| Molar mass | 156.228 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
Alkylated naphthalenes (methyl-, dimethyl-, and poly-methyl naphthalenes, thus including 2,6-DMN) are found in low concentrations in crude oil and coal tar. Separation is difficult, expensive, and requires a number of operations such as selective crystallization and adsorption, in addition to any isomerization reactions. Alternative routes to 2,6-DMN remains of interest.[2]
In the "alkenylation process" butadiene (1), o-xylene (2), and sodium-potassium alloy (3) are used, which react to form 5-(ortho-tolyl)pent-2-ene (OTP, 3).[3] OTP is subsequently cyclized to 1,5-dimethyltetraline (4). Dehydrogenation then provides 1,5-dimethylnaphthalene (1,5-DMN, 5). Finally, 1,5-DMN is isomerized to 2,6-DMN (6). In the final step, other mono-, di-, and tri-methylnaphthalenes are formed. More work is therefore required in separation of the mixture, which is done by selective crystallization.
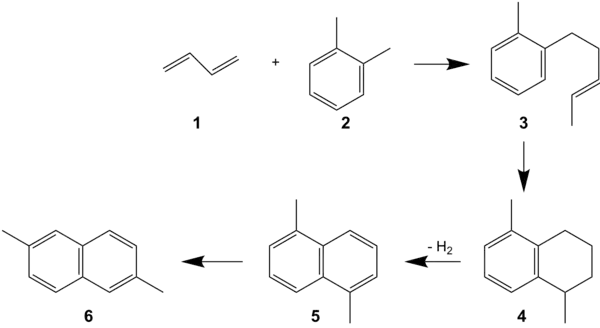 Synthesis of 2,6-dimethylnaphthalene by the alkenylation process
Synthesis of 2,6-dimethylnaphthalene by the alkenylation process
Applications
2,6-Dimethylnaphthalene is mainly used for the preparation of 2,6-naphthalenedicarboxylic acid by oxidation of 2,6-dimethylnaphthalene in the liquid phase. 2,6-Naphthalenedicarboxylic acid is a monomer for the production of high-performance polymers, in particular poly (ethylene-2,6-naphthalene dicarboxylate) or shorter polyethylene naphthalate (PEN).[4] That polyester is stronger and has a higher thermal resistance than the more frequently used polyethylene terephthalate (PET).
2,6-Dimethylnaphthalene undergoes ammoxidation to give the 2,6-dicyanonaphthalene, which can be hydrogenated to bis(aminomethyl)naphthalene, a precursor to dyes.[5]
References
- Collin, Gerd; Höke, Hartmut; Greim, Helmut (2003). "Naphthalene and Hydronaphthalenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_001.pub2.
- US patent 5004853, Paul T. Barger, Timothy J. Barder, David Y. Lin, Simon H. Hobbs, "Continuous process for the production of 2,6-dimethylnaphthalene", issued April 2, 1991
- US patent, Lawrence D. Lillwitz, Anne M. Karachewski, "Alkylation of alkylaromatics promoted by sonicated alkali metal", issued March 30, 1993
- Lillwitz, L. D. (2001). "Production of Dimethyl-2,6-Naphthalenedicarboxylate: Precursor to Polyethylene Naphthalate". Applied Catalysis A: General. 221 (1–2): 337–358. doi:10.1016/S0926-860X(01)00809-2.
- Pollak, Peter; Romeder, Gérard; Hagedorn, Ferdinand; Gelbke, Heinz-Peter (2000). "Nitriles". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_363.