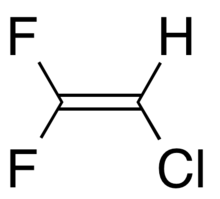
2-Chloro-1,1-difluoroethylene
2-Chloro-1,1-difluoroethene (also known as R 1122, u-HCFC-1122 or HCFO-1122) is a toxic unsaturated chlorofluorocarbon which can be written as CF2=CHCl. The HCFO portion of the name stands for hydrochlorofluoroolefin. Another constitutional isomer of it, 1-chloro-1,2-difluoroethylene, is known as HCFO-1122a.[2]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.006.024 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2HClF2 | |
| Molar mass | 98.48 g·mol−1 |
| Melting point | −138.5 °C (−217.3 °F; 134.7 K)[1] |
| Boiling point | −17.7 °C (0.1 °F; 255.5 K) |
| Related compounds | |
Other anions |
2-Bromo-1,1-difluoroethene |
Related compounds |
1-Chloro-1,2-difluoroethene (Z) and (E) isomers (R 1122a); |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Formation
One way to make HCFO-1122 by way of dehydrochlorination, is to heat HCFC-132b to 600°C, preferably with some carbon tetrachloride to get a 70% yield.
- CH2ClCClF2 → CHCl=CF2 + HCl[3]
A lower yield results if HCFC-132a is used:
- CHCl2CHF2 → CHCl=CF2 + HCl[3]
When trying to make HFC-134a from HCFC-133a, some HCFO-1122 is produced as a side product by way of dehydrofluorination.[3]
- CF3CH2Cl → CHCl=CF2 + HF[3] (low yield)
Properties
Shape and size
2-Chloro-1,1-difluoroethene has a flat shape with all atoms in the same plane. The two fluorine atoms can be distinguished by whether one is closer to hydrogen or chlorine F(H) or F(Cl). The bond lengths are: C=C 1.303 Å, C-F(H) 1.321 Å, C-F(Cl) 1.320 Å, C-Cl 1.731 Å, C-H 1.083 Å. For bond angles: ∠CCF(H) 123.4°, ∠CCF(Cl) 126.1°, ∠CCH 128.3°, ∠CCCl 121°.[4]
Spectrum
The infrared spectrum includes strong absorption bands at v10 at 751.1 cm−1, v5 at 971.5 and 970.2 cm−1, v4 at 1200.7 cm−1, v3 at 1341.7 cm−1, and v2 at 1747.5 cm−1.[5] Weaker absorption bands are at v7 at 578.0 and 577.4 cm−1, v6 at 844.9and 842.8 cm−1 and v1 at 3135.9 cm−1.[5] An estimate for radiative forcing potential is 0.098 W m2 ppbv−1 and global warming potential is between 1.5 and 4.5 on 100 year time frame.[5] The lifetime in Earth's atmosphere is only 10 to 30 days mitigating the effect of pollution.[5]
Occurrence
2-Chloro-1,1-difluoroethene may be a contaminant in HFC-134a.[6] It can form by the elimination of HCl or HF from other HCFCs like HCFC-133a. CF3CH2Cl → CF2=CHCl + HF. It can be removed from the mixture by various physical processes such as absorption, or chemical processes, that fluorinate, reduce or oxidise it.[7] An example specification for medical use of HFC-134a requires under 5 ppm of HCFC-1122.[8]
Humans that have been anesthetised by halothane, convert some in the body to 2-chloro-1,1-difluoroethene and then exhale it.[9]
Reactions
When irradiated by ultraviolet light at 192 nm, 2-Chloro-1,1-difluoroethene splits off hydrogen chloride to make a carbene: (difluorovinylidene) CF2=CHCl → CF2=C: + HCl.[10] Difluorovinylidene does not convert to difluoroacetylene (FC≡CF), but instead survives and reacts with other molecules.[11] Also HF can be eliminated to yield chlorofluoroacetylene (ClC≡CF).[12]
2-Chloro-1,1-difluoroethene can be removed from HFC-134a by oxidation with potassium permanganate.[13] Alternately oxidation can occur with hydrogen peroxide. Fluoridation can occur with HF with a chromium trioxide catalyst, producing CF3CH2Cl. With fluorine around −60°C it forms CF3CHClF.[14]
An argon complex with the molecule is known. The argon atom is out of the plane of the other atoms, on the side with the chlorine atom.[4]
When it is heated with cyclopentadiene at 170°C, bicyclic norbornene derivatives are produced.[15]
Wen heated with hydrogen, it is dechlorinated, and becomes the saturated 1,1-difluoroethane.[16]
Trichlorosilane reacts by adding across the double bond, mostly yielding trichloro-(2,2-difluoroethyl)silane. As 2-chloro-1,1-difluoroethene levels increase, more of trichloro-(2-chloro-2,2-difluoroethyl)silane and trichloro-(2-chloro-1,1-difluoroethyl)silane are produced.[17]
Use
2-Chloro-1,1-difluoroethene is an intermediate in the manufacture of fluorosurfactants, fluorine-containing textile finishing agents, organic silicon fluorine modified resins and other fine chemicals containing fluorine.[5]
References
- "SynQuest Labs, Inc". synquestlabs.com.
- "2903450010 1,1,1,2-Tétrafluoroéthane (HFC-134a)". www.douane.gouv.fr (in French).
- Sicard, Alexandre J.; Baker, R. Tom (9 September 2020). "Fluorocarbon Refrigerants and their Syntheses: Past to Present". Chemical Reviews. 120 (17): 9164–9303. doi:10.1021/acs.chemrev.9b00719. PMID 32809811. S2CID 221180753.
- Leung, Helen O.; Marshall, Mark D.; Messinger, Joseph P.; Knowlton, Gregory S.; Sundheim, Kathryn M.; Cheung-Lau, Jasmina C. (1 November 2014). "The microwave spectra and molecular structures of 2-chloro-1,1-difluoroethylene and its complex with the argon atom". Journal of Molecular Spectroscopy. 305: 25–33. Bibcode:2014JMoSp.305...25L. doi:10.1016/j.jms.2014.09.011.
- Pietropolli Charmet, Andrea; Ceselin, Giorgia; Stoppa, Paolo; Tasinato, Nicola (24 January 2022). "The Spectroscopic Characterization of Halogenated Pollutants through the Interplay between Theory and Experiment: Application to R1122". Molecules. 27 (3): 748. doi:10.3390/molecules27030748. PMC 8839295. PMID 35164013.
- 张, 永科; 赵, 景婵; 郭, 治安; 张, 彦凤; 韩, 冰 (2007). "医用HFC-134a中微量杂质的定性、定量分析" [Qualitative and Quantitative Analyses of Micro-impurities in Medical HFC-134a]. 应用化学. 24 (11). doi:10.3969/j.issn.1000-0518.2007.11.027.
- Manzer, L.E. (March 1992). "An overview of the commercial development of chlorofluorocarbon (CFC) alternatives". Catalysis Today. 13 (1): 13–22. doi:10.1016/0920-5861(92)80183-N.
- Noakes, Tim (December 2002). "Medical aerosol propellants". Journal of Fluorine Chemistry. 118 (1–2): 35–45. doi:10.1016/S0022-1139(02)00191-4.
- Sharp, J. Howard; Trudell, James R.; Cohen, Ellis N. (1 January 1979). "Volatile Metabolites and Decomposition Products of Halothane In Man". Anesthesiology. 50 (1): 2–8. doi:10.1097/00000542-197901000-00002. PMID 760598. S2CID 27245452.
- Huang, Yibo; Gordon, Robert J. (22 January 1997). "The ultraviolet photodissociation dynamics of 2-chloro-1,1-difluoroethylene". The Journal of Chemical Physics. 106 (4): 1418–1420. Bibcode:1997JChPh.106.1418H. doi:10.1063/1.473290.
- Brahms, John C.; Dailey, William P. (May 1990). "Difluoropropadienone as a source of difluorovinylidene and difluorodiazoethene". Journal of the American Chemical Society. 112 (10): 4046–4047. doi:10.1021/ja00166a056.
- Martínez-Núñez, Emilio; Vázquez, Saulo (8 March 2005). "Quasiclassical trajectory calculations on the photodissociation of CF2CHCl at 193nm: Product energy distributions for the HF and HCl eliminations". The Journal of Chemical Physics. 122 (10): 104316. Bibcode:2005JChPh.122j4316M. doi:10.1063/1.1859276. PMID 15836324.
- Manzer, L. E. (6 July 1990). "The CFC-Ozone Issue: Progress on the Development of Alternatives to CFCs". Science. 249 (4964): 31–35. Bibcode:1990Sci...249...31M. doi:10.1126/science.249.4964.31. PMID 17787623. S2CID 11822992.
- Guglielmo, Giorgio; Gambaretto, Giampaolo (22 November 1994). "Process for purifying fluoroethanes and chlorofluoroethanes".
- Jacobson, Barry M.; Bartlett, Paul D. (March 1973). "Cycloaddition. XV. Competing mechanisms in the reactions of cyclopentadiene with trifluoroethylene and 2-chloro-1,1-difluoroethylene". The Journal of Organic Chemistry. 38 (5): 1030–1041. doi:10.1021/jo00945a035.
- Lacher, J. R.; Kianpour, A.; Oetting, F.; Park, J. D. (1956). "Reaction calorimetry. The hydrogenation of organic fluorides and chlorides". pp. 1500–1508.
- Bevan, William I.; Haszeldine, Robert N.; Middleton, John; Tipping, Anthony E. (1974). "Polyfluoroalkyl compounds of silicon. Part XII. Reactions of trichlorosilane with 2-chloro- and 2-bromo-1,1-difluoroethylene". Journal of the Chemical Society, Dalton Transactions (21): 2305. doi:10.1039/DT9740002305.