2-Hydroxy-4-(methylthio)butyric acid
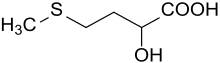
2-Hydroxy-4-(methylthio)butyric acid is an organic compound with the structural formula CH3SCH2CH2CH(OH)CO2H. It is a white solid. In terms of functional groups, the molecule is a α-hydroxy carboxylic acid and a thioether. The compound is structurally related to the amino acid methionine by replacement of the amine with a hydroxy group.
 | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.008.659 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H10O3S | |
| Molar mass | 150.19 g·mol−1 |
| Appearance | colorless or white solid |
| Hazards | |
| GHS labelling:[1] | |
  | |
| Danger | |
| H315, H318, H412 | |
| P264, P264+P265, P273, P280, P302+P352, P305+P354+P338, P317, P321, P332+P317, P362+P364, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The compound is produced commercially in racemic form from acrolein by conjugate addition of methanethiol followed by formation and hydrolysis of a cyanohydrin.[2] it is used as a substitute for methionine in animal feed.[3]
In nature, the compound is also an intermediate in the biosynthesis of 3-dimethylsulfoniopropionate, precursor to natural dimethyl sulfide.[4]
References
- "2-Hydroxy-4-(methylthio)butyric acid". pubchem.ncbi.nlm.nih.gov. Retrieved 28 April 2022.
- Rey, Patrick; Rossi, Jean-Christophe; Taillades, Jacques; Gros, Georges; Nore, Olivier (2004). "Hydrolysis of Nitriles Using an Immobilized Nitrilase: Applications to the Synthesis of Methionine Hydroxy Analogue Derivatives". Journal of Agricultural and Food Chemistry. 52 (26): 8155–8162. doi:10.1021/jf048827q. PMID 15612811.
- Lemme, A.; Hoehler, D.; Brennan, JJ; Mannion, PF (2002). "Relative Effectiveness of Methionine Hydroxy Analog Compared to DL-Methionine in Broiler Chickens". Poultry Science. 81 (6): 838–845. doi:10.1093/ps/81.6.838. PMID 12079051.
- Curson, Andrew R. J.; Liu, Ji; Bermejo Martínez, Ana; Green, Robert T.; Chan, Yohan; Carrión, Ornella; Williams, Beth T.; Zhang, Sheng-Hui; Yang, Gui-Peng; Bulman Page, Philip C.; Zhang, Xiao-Hua; Todd, Jonathan D. (2017). "Dimethylsulfoniopropionate Biosynthesis in Marine Bacteria and Identification of the Key Gene in this Process" (PDF). Nature Microbiology. 2 (5): 17009. doi:10.1038/nmicrobiol.2017.9. PMID 28191900. S2CID 21460292.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.