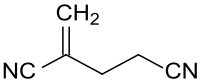
2-Methyleneglutaronitrile
2-Methylene glutaronitrile is a dimerization product of acrylonitrile and a starting material for di- and triamines, for the biocide 2-bromo-2-(bromomethyl)pentanedinitrile and for heterocycles, such as 3-cyanopyridine.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylidenepentanedinitrile | |
| Other names
2,4-dicyano-1-butene 2-Methylenepentanedinitrile | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.014.902 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H6N2 | |
| Molar mass | 106.13 g·mol−1 |
| Appearance | clear, colourless[1] liquid |
| Density | 0.976 g·cm−3 (25 °C)[2] |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H312, H332 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P312, P322, P330, P363, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation
2-Methylene glutaronitrile is a side-product in the production of hexanedinitrile which is used (after hydrogenation to 1,6-diaminohexane) as a key component for engineering polymers such as the polyamides (PA 66) or polyurethanes. Hexanedinitrile can be industrially produced by electrochemical hydrodimerisation or by catalytic dimerization of acrylonitrile.
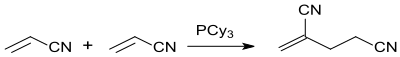
A catalytic tail-tail dimerization of two acrylonitrile molecules forms hexanedinitrile:
Also head-to-tail dimerization can occur in the process. In the presence of tricyclohexylphosphine (PCy3) a yield of up to 77% 2-methylene glutaronitrile can be obtained:[3]
Metal halides (such as zinc chloride[4][5] or aluminium chloride[6]) are used with tertiary amines (such as triethylamine) as catalysts for the dimerization. Crude yields of up to 84% are achieved.[4] Often, significant amounts of product are lost during the work-up (e. g. extraction and distillation) because of the tendency to polymerization of 2-methylene glutaronitrile.
In addition to the linear dimerization products 1,4-dicyano-2-butene and 1,4-dicyano-3-butene (obtained as cis-trans isomer mixtures) usually also other oligomers (and polymers) of acrylonitrile are formed. During the electrochemical hydrooligomerization of acrylonitrile, these are trimers, such as 1,3,6- and 1,3,5-tricyanohexane or tetramers, such as 1,3,6,8- and 1,3,5,8-tetracyanooctane.[7] The reaction of acrylonitrile with tributylphosphine affords 2-methyleneglutaronitrile in a modest yield of about 10% after fractional distillation.[8] The DABCO-catalyzed acrylonitrile dimerization of 2,4-dicyano-1-butene after 10 days at room temperature is with 40% yield similarly inefficient.[9]
Use
The earlier patent literature describes processes for the isomerization of 2-methylene glutaronitrile to 1,4-dicyanobutenes as hexanedinitrile precursors, which became obsolete with the optimization of the electrochemical hydrodimerization of acrylonitrile to hexanedinitrile.[10]
The electrochemical hydrodimerization of 2-methylene glutaronitrile produces 1,3,6,8-tetracyanooctane.[8]
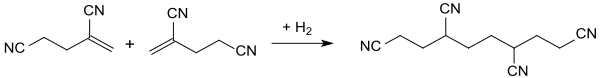
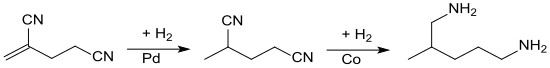
In the hydrogenation of 2-methylene glutaronitrile in the presence of palladium on carbon, hydrogen is attached to the double bond and 2-methylglutaronitrile is obtained in virtually quantitative yield.[11]
The hydrogenation of the nitrile groups requires more severe conditions and the presence of ammonia or amines to suppress the formation of secondary amines. This second hydrogenation step is carried out with Raney-cobalt as the hydrogenation catalyst to give 2-methyl-1,5-pentanediamine in 80% yield.[12]
Hydrogenation of 2-methylene glutaronitrile in the presence of ammonia with manganese-containing sodium oxide-doped cobalt catalyst (at 80 to 100 °C and pressures of 200 atm in a tubular reactor) leads to the addition of ammonia to the double bond and directly converts the compound to 2-aminomethyl-1,5-pentanediamine with yields of 66%.[13]
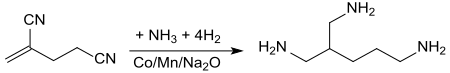
The branched triamine can be used in epoxides and polyurethanes.
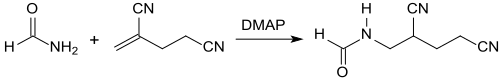
2-Methylenglutaronitrile reacts with methanamide upon catalysis with 4-(dimethylamino)-pyridine (DMAP) at 60 °C in 47% yield to give 1-(N-methanoylamino)-2,4-dicyanobutane, from which α- aminomethylglutaric acid is formed by subsequent hydrolysis.[14]
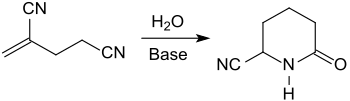
Heating 2-methyleneglutaronitrile with an alkaline ion exchanger, pyridine and water to 150 °C in an autoclave yields the lactam 5-cyano-2-piperidone in 80% yield.[15]
2-Methylene glutaronitrile can be polymerized to various homo- and copolymers via anionic polymerization with sodium cyanide, sodium in liquid ammonia or with butyllithium. However, the polymers are formed only in low yields and show unsatisfactory properties such as intrinsic viscosities and poor mechanical properties.[16]
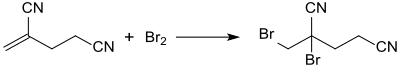
The main use of 2-methyleneglutaronitrile is as starting material for the broad-spectrum biocide 2-bromo-2-(bromomethyl)pentanedinitrile (methyldibromo-glutaronitrile),[17] which is formed in virtually quantitative yield by the addition of bromine to the double bond.[18]
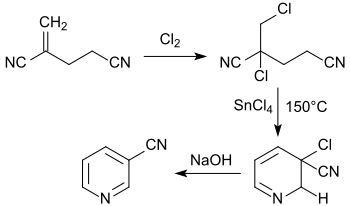
From the chlorine-analogous 2-chloro-2-(chloromethyl)pentenenitrile, 3-cyanopyridine is obtained by heating to 150 °C with tin(IV)chloride.[17]
References
- "2-Methyleneglutaronitrile 1572-52-7 | TCI America". www.tcichemicals.com. Archived from the original on 2018-01-15. Retrieved 2018-01-14.
- Sigma-Aldrich Co., product no. 125547.
- L. Yu; et al. (2014), "Practical and scalable preparation of 2-methyleneglutaronitrile via an efficient and highly selective head-to-tail dimerization of acrylonitrile catalysed by low-loading of tricyclohexylphosphine", RSC Adv., vol. 4, pp. 19122–19126, doi:10.1039/C4RA02810D
- US 3733351, Y. Watanabe, M. Takeda, "Production of 2-methylene-glutaronitrile", published 1973-05-15, assigned to Mitsubishi Petrochemical Co.
- US 4422981, H. Omori, M. Takeda, K. Fujita, M. Kataoka, "Process for production of 2-methyleneglutaronitrile", published 1983-12-27, assigned to Mitsubishi Petrochemical Co.
- US 3956358, O.T. Onsager, "Dimerization method", published 1976-05-11, assigned to Halcon International, Inc.
- M.M. Baizer; J.D. Anderson (1965), "Electrolytic reductive coupling. VII. A new class of acrylonitrile oligomers", J. Org. Chem., vol. 30, no. 5, pp. 1351–1356, doi:10.1021/jo01016a003
- M.M. Baizer; J.D. Anderson (1965), "Electrolytic reductive coupling. VIII. Utilization and a new preparation of α-methyleneglutaronitrile", J. Org. Chem., vol. 30, no. 5, pp. 1357–1360, doi:10.1021/jo01016a004
- D. Basavaiah; V.V.L. Gowriswari; T.K. Barathi (1987), "DABCO catalyzed dimerization of α, β-unsaturated ketones and nitriles", Tetrahedron Lett., vol. 28, no. 39, pp. 4591–4592, doi:10.1016/S0040-4039(00)96573-0
- US 3795694, O.T. Onsager, "Preparation of cyano compounds", published 1974-03-05, assigned to Halcon International, Inc.
- US 3350439, J. Feldman, M. Thomas, "Process for preparing aminoalkanenitriles", published 1967-10-31, assigned to National Destillers and Chemical Corp.
- US 3408397, J. Feldman, M. Thomas, "Methyl pentamethylene diamine process", published 1967-10-31, assigned to National Destillers and Chemical Corp.
- EP 1028104, K. Fischer, F. Richter, A. Bazanov, A. Timofeev, N. Zubritskaya, G. Smirnova, "Verfahren zur Herstellung von 2-Aminomethyl-1,5-pentandiamin", published 2000-08-16, assigned to Bayer AG
- EP 0336185, H.-J. Scholl, "1-(N-Formylamino)-2,4-dicyanobutan und ein Verfahren zu dessen Herstellung", published 1989-10-11, assigned to Bayer AG
- US 3666766, J.B. Pedigo, J. Feldman, I.A. Kereszies, "Selective hydrolysis and cyclization of unsaturated nitriles", published 1972-05-30, assigned to National Distillers and Chemical Corp.
- US 3451977, J.M. Hoyt, K. Koch, "Process for polymerizing 2-methylene glutaronitrile", published 1969-06-24, assigned to National Distillers and Chemical Corp.
- US 3644380, R. Harmetz, R.J. Tull, "Preparation of 3-cyanopyridine", published 1972-02-22, assigned to Merck & Co., Inc.
- US 3929858, R.D. Swigert, "Method for preparing 2-bromo-2-bromomethyl-glutaronitrile", published 1975-12-30, assigned to Merck & Co., Inc.