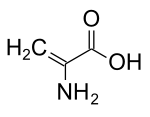
Dehydroalanine
Dehydroalanine (Cα,β-didehydroalanine, α,β-di-dehydroalanine, 2-aminoacrylate, or 2,3-didehydroalanine) is a dehydroamino acid. It does not exist in its free form, but it occurs naturally as a residue found in peptides of microbial origin.[1] As an amino acid residue, it is unusual because it has an unsaturated backbone.[2]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Aminoprop-2-enoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H5NO2 | |
| Molar mass | 87.08 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Structure and reactivity
Like most primary enamines, dehydroalanine is unstable. Dehydroalanine hydrolyzes to pyruvate.
N-Acylated derivatives of dehydroalanine, such as peptides and related compounds, are stable. For example, methyl 2-acetamidoacrylate is the N-acetylated derivative of the ester. As a residue in a peptide, it is generated by a post translational modification. The required precursors are serine or cysteine residues, which undergo enzyme-mediated loss of water and hydrogen sulfide, respectively.
Most amino acid residues are unreactive toward nucleophiles, but those containing dehydroalanine or some other dehydroamino acids are exceptions. These are electrophilic due to the α,β-unsaturated carbonyl,[2] and can, for example, alkylate other amino acids. This activity has made DHA useful synthetically to prepare lanthionine.
Occurrence
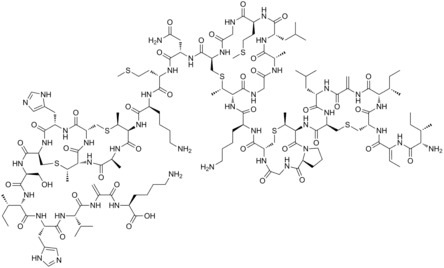
The dehydroalanine residue was first detected in nisin, a cyclic peptide with antimicrobial activity.[2] Dehydroalanine is also present in some lantibiotics and microcystins.
DHA can be formed from cysteine or serine by simple base catalysis without the need for an enzyme, which can happen during cooking and alkaline food preparation processes. It can then alkylate other amino acid residues, such as lysine, forming lysinoalanine cross-links and racemization of the original alanine. The resulting proteins have lower nutritional quality for some species but higher nutritional quality for others. Some lysinoalanines may also cause kidney enlargement in rats.[3]
Many dehydroalanine-containing peptides are toxic.[2]
A dehydroalanine residue was long thought to be an important electrophilic catalytic residue in histidine ammonia-lyase and phenylalanine ammonia-lyase enzymes, but the active residue was later found instead to be a different unsaturated alanine derivative — 3,5-dihydro-5-methyldiene-4H-imidazol-4-one — that is even more electrophilic.[4][5]
Chemical synthesis
N-Acyl dehydroalanine derivatives have been synthesized by dehydration of serines using a tert-butoxycarbonate leaving group,[6] or by conversion of Cysteine derivatives using various reagents for the elimination of the Thiol-group.[7]
Newest methods allow the gram-scale synthesis of various protected dehydroamino acids by electrochemical oxidation of the respective amino acid derivative in methanol, followed by acid-catalyzed elimination of methanol.[8]
References
- Downs, DM; Ernst, DC (April 2015). "From microbiology to cancer biology: the Rid protein family prevents cellular damage caused by endogenously generated reactive nitrogen species". Molecular Microbiology. 96 (2): 211–9. doi:10.1111/mmi.12945. PMC 4974816. PMID 25620221.
- Siodłak, Dawid (2015). "α,β-Dehydroamino Acids in Naturally Occurring Peptides". Amino Acids. 47 (1): 1–17. doi:10.1007/s00726-014-1846-4. PMC 4282715. PMID 25323736.
- Friedman, Mendel (1999). "Lysinoalanine in food and in antimicrobial proteins". In Jackson, Lauren S.; Knize, Mark G.; Morgan, Jeffrey N. (eds.). Impact of Processing on Food Safety. Advances in Experimental Medicine and Biology. Vol. 459. Springer. pp. 145–159. doi:10.1007/978-1-4615-4853-9_10. ISBN 978-1-4615-4853-9. PMID 10335374.
- Rétey, János (2003). "Discovery and role of methylidene imidazolone, a highly electrophilic prosthetic group". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 1647 (1–2): 179–184. doi:10.1016/S1570-9639(03)00091-8. PMID 12686130.
- Calabrese JC, Jordan DB, Boodhoo A, Sariaslani S, Vannelli T (September 2004). "Crystal structure of phenylalanine ammonia lyase: multiple helix dipoles implicated in catalysis". Biochemistry. 43 (36): 11403–16. doi:10.1021/bi049053+. PMID 15350127.
- Ferreira, Paula M. T.; Maia, Hernâni L. S.; Monteiro, Luís S.; Sacramento, Joana (1999). "High yielding synthesis of dehydroamino acid and dehydropeptide derivatives". Journal of the Chemical Society, Perkin Transactions 1 (24): 3697–3703. doi:10.1039/a904730a. hdl:1822/2188.
- Chalker, Justin M.; Gunnoo, Smita B.; Boutureira, Omar; Gerstberger, Stefanie C.; Fernández-González, Marta; Bernardes, Gonçalo J. L.; Griffin, Laura; Hailu, Hanna; Schofield, Christopher J.; Davis, Benjamin G. (2011). "Methods for converting cysteine to dehydroalanine on peptides and proteins". Chemical Science. 2 (9): 1666. doi:10.1039/c1sc00185j. ISSN 2041-6520.
- Gausmann, Marcel; Kreidt, Nadine; Christmann, Mathias (2023-04-07). "Electrosynthesis of Protected Dehydroamino Acids". Organic Letters. 25 (13): 2228–2232. doi:10.1021/acs.orglett.3c00403. ISSN 1523-7060. PMID 36952622. S2CID 257716096.