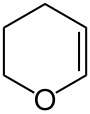
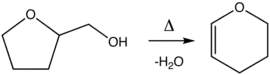3,4-Dihydropyran
3,4-Dihydropyran (DHP) is a heterocyclic compound with the formula C5H8O. The six-membered C5O ring has the unsaturation adjacent to oxygen. The isomeric 3,6-dihydropyran has a methylene separating the double bond and oxygen. DHP is used for protecting group for alcohols. It is a colorless liquid.[1]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3,4-Dihydro-2H-pyran | |
| Other names
2,3-Dihydro-4H-pyran, DHP | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.465 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 2376 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H8O | |
| Molar mass | 84.118 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.922 g/mL |
| Melting point | −70 °C (−94 °F; 203 K) |
| Boiling point | 86 °C (187 °F; 359 K) |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H225, H315, H317, H319 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P272, P280, P302+P352, P303+P361+P353, P305+P351+P338, P321, P332+P313, P333+P313, P337+P313, P362, P363, P370+P378, P403+P235, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Preparation
Dihydropyran is prepared by the dehydration of tetrahydrofurfuryl alcohol over alumina at 300–400 °C.[2] THFA is itself prepared from tetrahydro-2-furoic acid.
Reactions
In organic synthesis, the 2-tetrahydropyranyl (THP) group is used as a protecting group for alcohols.[3][4] Reaction of the alcohol with DHP forms a THP ether, protecting the alcohol from a variety of reactions. The alcohol can later be restored by acidic hydrolysis, concomitant with formation of 5-hydroxypentanal.[5]
 Protection of an alcohol as THP ether followed by its deprotection. Both steps require acid catalysts.
Protection of an alcohol as THP ether followed by its deprotection. Both steps require acid catalysts.
See also
References
- Paul Ch. Kierkus (2001). "3,4‐Dihydro‐2H‐pyran". eEROS. doi:10.1002/047084289X.rd230.
- R. L. Sawyer and D. W. Andrus (1955). "2,3-Dihydropyran". Organic Syntheses.; Collective Volume, vol. 3, p. 276
- R. A. Earl L. B. Townsend (1990). "Methyl 4-Hydroxy-2-butynoate". Organic Syntheses.; Collective Volume, vol. 7, p. 334
- Arthur F. Kluge (1990). "Diethyl [(2-Tetrahydropyranyloxy)methyl]phosphonate". Organic Syntheses.; Collective Volume, vol. 7, p. 160
- Wuts, Peter G. M.; Greene, Theodora W. (2006). "Protection for the Hydroxyl Group, Including 1,2‐ and 1,3‐Diols". Greene's Protective Groups in Organic Synthesis (4th ed.). pp. 16–366. doi:10.1002/9780470053485.ch2. ISBN 9780470053485.