3-Hydroxykynurenine
3-Hydroxykynurenine is a metabolite of tryptophan, which filters UV light in the human lens.[1] It is one of two pigments identified as responsible for the goldenrod crab spider's (Misumena vatia) yellow coloration.
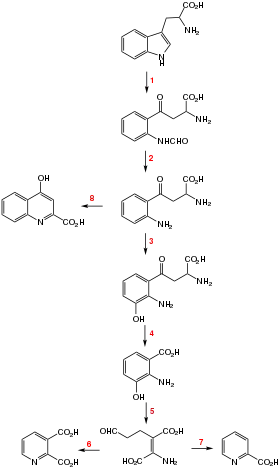
The Kynurenine pathway, which connects quinolinic acid to tryptophan. The pathway is named for the first intermediate, kynurenine, which is a precursor to kynurenic acid and 3-hydroxykynurenine.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-Amino-4-(2-amino-3-hydroxyphenyl)-4-oxobutanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
| MeSH | 3-hydroxykynurenine |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C10H12N2O4 | |
| Molar mass | 224.21 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Malina, HZ; Martin, XD (1995). "Deamination of 3-hydroxykynurenine in bovine lenses: a possible mechanism of cataract formation in general". Graefes Arch Clin Exp Ophthalmol. 233 (1): 38–44. doi:10.1007/bf00177784. PMID 7721122. S2CID 25414197.
- Schwarcz, Robert; John P. Bruno; Paul J. Muchowski; Hui-Qiu Wu (July 2012). "Kynurenines in the Mammalian Brain: When Physiology Meets Pathology". Nature Reviews Neuroscience. 13 (7): 465–477. doi:10.1038/nrn3257. PMC 3681811. PMID 22678511.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.