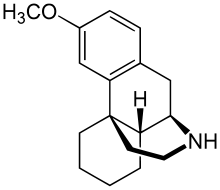
3-Methoxymorphinan
3-Methoxymorphinan is a levomethorphan metabolite that has been shown to produce local anesthetic effects.[1] It is the CYP3A4 metabolite of dextromethorphan,[2] and is itself metabolized by the liver enzyme CYP2D6.[3]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.014.764 |
| Chemical and physical data | |
| Formula | C17H23NO |
| Molar mass | 257.377 g·mol−1 |
| |
| | |
References
- Hou, Chia-Hui; Tzeng, Jann-Inn; Chen, Yu-Wen; Lin, Ching-Nan; Lin, Mao-Tsun; Tu, Chieh-Hsien; Wang, Jhi-Joung (2006). "Dextromethorphan, 3-methoxymorphinan, and dextrorphan have local anaesthetic effect on sciatic nerve blockade in rats". European Journal of Pharmacology. 544 (1–3): 10–6. doi:10.1016/j.ejphar.2006.06.013. PMID 16844109.
- Min, David I.; Ku, Yi-Min; Vichiendilokkul, Aungkana; Fleckenstein, Lawrence L. (1999). "A Urine Metabolic Ratio of Dextromethorphan and 3-Methoxymorphinan as a Probe for CYP3A Activity and Prediction of Cyclosporine Clearance in Healthy Volunteers". Pharmacotherapy. 19 (6): 753–9. doi:10.1592/phco.19.9.753.31536. PMID 10391422.
- Strauch K, Lutz U, Bittner N, Lutz WK (August 2009). "Dose-response relationship for the pharmacokinetic interaction of grapefruit juice with dextromethorphan investigated by human urinary metabolite profiles". Food and Chemical Toxicology. 47 (8): 1928–35. doi:10.1016/j.fct.2009.05.004. PMID 19445995.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.