4,4'-Methylenebis(2-chloroaniline)
4,4′-Methylenebis(2-chloroaniline) (also known as MOCA, MBOCA, and bisamine) is a substance used as a curing agent in polyurethane production.[3] MOCA is an aromatic amine which is structurally similar to benzidine, a known human bladder carcinogen. MOCA has been shown to cause hepatomas in mice and rats, lung and mammary carcinomas in rats and bladder cancer in dogs. It is a proven human carcinogen standing on the WHO List of IARC Group 1 carcinogens, with a current threshold limit value of 0.01 ppm in the industrial atmosphere. Animal studies have resulted in tumor growth in the liver, lung, and bladder.[1]
 | |
| Names | |
|---|---|
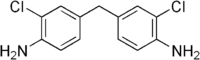
| Preferred IUPAC name
4,4′-Methylenebis(2-chloroaniline) | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.654 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H12Cl2N2 | |
| Molar mass | 267.15 g·mol−1 |
| Appearance | Tan-colored pellets or flakes[1] |
| Odor | faint, amine-like[1] |
| Density | 1.44 g/cm3[1] |
| Melting point | 104 to 109 °C (219 to 228 °F; 377 to 382 K)[2] |
| insoluble | |
| Vapor pressure | 0.00001 mmHg (20 °C)[1] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
potential occupational carcinogen[1] |
| NFPA 704 (fire diamond) | |
| Flash point | 203 °C (397 °F; 476 K) |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
none[1] |
REL (Recommended) |
Ca TWA 0.003 mg/m3 [skin][1]4,4′-Methylen-bis(2-chloranilin), kurz MOCA, ist eine chemische Verbindung, die seit 1954 als Vernetzer von TDI-Polyurethanen (PU) eingesetzt wird.
Schnelle Fakten: Strukturformel, Allgemeines ... Darstellung 4,4′-Methylen-bis(2-chloranilin) wird kommerziell durch Umsetzung von Formaldehyd mit ortho-Chloroanilin hergestellt. Technische Produkte enthalten typischerweise 90–92 % 4,4′-Methylen-bis(2-chloranilin). Gesundheitsgefahren MOCA stand seit langem unter dem Verdacht, krebserregend zu sein. Die Internationale Agentur für Krebsforschung der WHO stuft u. a. auf Grundlage sicherer Erkenntnisse über den Mechanismus der Krebsentstehung bei Tieren MOCA seit 2012 in die höchste Gruppe 1: "karzinogen für Menschen". Die EU-Kommission hat deshalb im April 2018 vorgeschlagen, einen neuen Grenzwert für MOCA gesetzlich in der dritten Änderung der Richtlinie über Karzinogene und Mutagene (KM-Richtlinie) festzulegen, um damit Arbeitnehmer besser schützen zu können. |
IDLH (Immediate danger) |
Ca [N.D.][1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Employee exposure is often monitored by measurement of urinary MOCA in free and/or conjugated form.[4] The best currently available indicator of absorption of MOCA is urinary total MOCA estimations based on spot creatinine corrected urines. Although this method is not without limitations, that is unmetabolised MOCA is measured and dose-response curves are lacking, its use is a reasonable means of monitoring the effectiveness of engineering controls, personal protective equipment and work practices including education. MOCA levels are usually higher at the end of the shift and reflect exposure over the preceding two to three days. The biological half-life of MOCA in urine is approximately 23 hours.
It is a weak base with a slight odor and is reactive to active metals such as sodium, potassium, magnesium and zinc.[1]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0411". National Institute for Occupational Safety and Health (NIOSH).
- "4,4'-Methylenebis(2-chloroaniline)". TCI Chemicals. Retrieved March 17, 2014.
- RTK HSFS 1250
- 4,4'-Methylene bis (2-chloroaniline) Health Guideline, Occupational Safety and Health Administration
