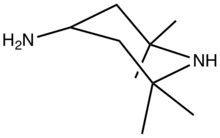
4-Amino-2,2,6,6-tetramethylpiperidine
4-Amino-2,2,6,6-tetramethyl-4-piperidine is an organic compound with the formula H2NCH(CH2CMe2)2NH (where Me = CH3). Classified as a diamine, it is a colorless oily liquid.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,2,6,6-Tetramethylpiperidin-4-amine | |
| Other names
2,2,6,6-Tetramethyl-4-aminopiperidine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.048.345 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H20N2 | |
| Molar mass | 156.273 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.8966 g/cm3 |
| Melting point | 17 °C (63 °F; 290 K) |
| Boiling point | 188.5 °C (371.3 °F; 461.6 K) |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H290, H302, H314, H412 | |
| P234, P260, P264, P270, P273, P280, P301+P312, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P330, P363, P390, P404, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The compound is an intermediate in the preparation of Bobbitt's salt, an oxidant used in organic synthesis. It is prepared by the reductive amination of the corresponding ketone:[1]
- OC(CH2CMe2)2NH + NH3 + H2 → H2NCH(CH2CMe2)2NH + H2O
Compound Properties
Boiling point is 188.5 °C [2] Melting point is 17 °C. [3] Density is 0.8966 g/cm3 @ Temp: 20 °C [4]
Toxicity
A study by Truda et al, has reported the median lethal dose LD(50) as 906mg/kg in rats. .[5]
References
- Nabyl Merbouh; James M. Bobbitt; Christian Brückner (2004). "Preparation of Tetramethylpiperdine-1-oxoammonlum Salts and Their Use as Oxidants in Organic Chemistry. A Review". Organic Preparations and Procedures International. 36: 1–31. doi:10.1080/00304940409355369. S2CID 98117103.
- [PhysProp data were obtained from: https://commonchemistry.cas.org/detail?cas_rn=36768-62-4]
- [PhysProp data were obtained from: https://commonchemistry.cas.org/detail?cas_rn=36768-62-4]
- [PhysProp data were obtained from: https://commonchemistry.cas.org/detail?cas_rn=36768-62-4]
- [ Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 28(5), Pg. 53, 1984]
Related compounds
Further reading
- C. McFarland; D. Vicic; A. Debnath (2006). "Rapid Microwave-Assisted Syntheses of Derivatives of HIV-1 Entry Inhibitors". Synthesis. 2006 (5): 807–812. doi:10.1055/s-2006-926339.
- L. Tilley; J. Bobbitt; S. Murray; C. Camire (2013). "A Revised Preparation of (4-Acetamido-2,2,6,6-tetramethylpiperidin-1-yl)oxyl and 4-Acetamido-2,2,6,6-tetramethyl-1-oxopiperidinium Tetrafluoroborate: Reagents for Stoichiometric Oxidations of Alcohols". Synthesis. 45 (3): 326–329. doi:10.1055/s-0032-1317861.
- A. Germanova; L. Aizvert; A. Shidlovskaia; L. Melnikova; M. Bidevkina (1984). "Comparative characteristics of the toxicity, safety and nature of the biological effect on the body of 4-hydroxy-2,2,6,6-tetramethylpiperidine and 4-amino-2,2,6,6-tetramethylpiperidine". Gig Tr Prof Zabol (5): 53–54. PMID 6745687.
- M. Gergely; A. Takacs; L.Kollar (2016). "4-Amino-TEMPO as N-Nucleophile in Palladium-Catalyzed Aminocarbonylation". Heterocyclic Chemistry. 54 (1): 634–640. doi:10.1002/jhet.2635.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.