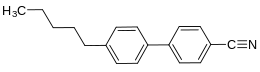
4-Cyano-4'-pentylbiphenyl
4-Cyano-4'-pentylbiphenyl is a commonly used nematic liquid crystal with the chemical formula C18H19N. It frequently goes by the common name 5CB. 5CB was first synthesized by George William Gray, Ken Harrison, and J.A. Nash at the University of Hull in 1972 and at the time it was the first member of the cyanobiphenyls.[2][3] The liquid crystal was discovered after Gray's group received a grant from the UK Ministry of Defence to find a liquid crystal that had liquid crystal phases near room temperature with the specific intention of using them in liquid crystal displays. The molecule is about 20 Å long. The liquid crystal 5CB undergoes a phase transition from a crystalline state to a nematic state at 22.5 °C and it goes from a nematic to an isotropic state at 35.0 °C.[1]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
4′-Pentyl[1,1′-biphenyl]-4-carbonitrile | |
| Other names
4'-Amyl-4-biphenylcarbonitrile, 4'-Pentyl-4-biphenylcarbonitrile, 4-Amyl-4'-cyanobiphenyl | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.050.068 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H19N | |
| Molar mass | 249.357 g·mol−1 |
| Appearance | Colorless if isotropic or cloudy white if nematic |
| Density | 1.022 g/cm3[1] |
| Melting point | 22.5[1] °C (72.5 °F; 295.6 K) |
Refractive index (nD) |
n// = 1.71, n⟂ = 1.53[1] |
| Viscosity | 28 mPa·s[1] |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H312, H315, H319, H332, H335 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Production
5CB is produced by modifying biphenyl in a linear manner. First Br2 is added to the biphenyl to introduce a bromine atom to the end of the moiety. Next aluminium chloride and C4H9COCl is added to the sample, followed by the addition of potassium hydroxide and NH2NH2. By this point the molecule will have a bromine atom on one end of the rigid core and C5H11 on the other end. Finally, introduction of copper(I) cyanide and DMF results in the removal of the bromine and its replacement with CN, yielding 5CB.[3]
References
- Zannoni, Claudio (2022). Liquid crystals and their computer simulations. Cambridge, United Kingdom. p. 15. ISBN 978-1-108-53963-0.
{{cite book}}: CS1 maint: location missing publisher (link) - Gray, George William; Harrison, Ken J.; Nash, J A. (1973). "New family of nematic liquid crystals for displays". Electronics Letters. 9 (6): 130–131. Bibcode:1973ElL.....9..130G. doi:10.1049/el:19730096.
- Collings, Peter J.; Hird, Michael (1997). Gray, George William; Goodby, J. W.; Fukuda, A. (eds.). Introduction to Liquid Crystals: Chemistry and Physics. Taylor and Francis Ltd. pp. 53, 151–152. ISBN 0-7484-0643-3.
Further reading
- Gray, George William (January 1998). "Reminiscences from a life with liquid crystals". Liquid Crystals. 24 (1): 5–14. doi:10.1080/026782998207523.
- Seo, D. S.; Matsuda, H.; Oh-Ide, T.; Kobayashi, S. (1993). "Alignment of Nematic Liquid Crystal(5CB) on the Treated Substrates: Characterization of Orientation Films, Generation of Pretilt Angles, and Surface Anchoring Strength". Molecular Crystals and Liquid Crystals. 224 (1): 13–31. doi:10.1080/10587259308032475.
- Vill, Volkmar (January 1998). "Grey levels in the history of liquid crystals". Liquid Crystals. 24 (1): 21–24. doi:10.1080/026782998207541.
External links
- Space Filling Model of 5CB
- "Liquid Crystal Displays (1973-1982)". Malvern Radar and Technology History Society. 2016.
