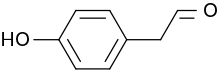
4-Hydroxyphenylacetaldehyde
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(4-Hydroxyphenyl)acetaldehyde | |
| Other names
p-Hydroxyphenylacetaldehyde | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.216.847 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H8O2 | |
| Molar mass | 136.150 g·mol−1 |
| Appearance | White solid |
| Melting point | 118 °C (244 °F; 391 K) |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H312, H315, H319, H332, H335 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
4-Hydroxyphenylacetaldehyde, also known as p-hydroxyphenylacetaldehyde, is a natural product with the formula HOC6H4CH2CHO. It is a derivative of phenylacetaldehyde and occurs as a white solid at room temperature.[1]
Synthesis
4-Hydroxyphenylacetaldehyde can be synthesized from a parsley tyrosine decarboxylase (L-tyrosine).[2]
Occurrence
4-Hydroxyphenylacetaldehyde is produced from the metabolism of tyramine by monoamine oxidase (MAO) enzymes in humans and the tyramine oxidase (tynA) enzyme in Escherichia coli.[3][4] In both species, it is subsequently metabolized into 4-hydroxyphenylacetate by aldehyde dehydrogenase (ALDH) enzymes in humans and the phenylacetaldehyde dehydrogenase (feaB) enzyme in E. coli.[3][4][5]
The condensation of 4-hydroxyphenylacetaldehyde and dopamine is a key step in the biosynthesis of benzylisoquinoline alkaloids. These natural products include berberine and morphine.[6]
References
- "4-Hydroxyphenylacetaldehyde". PubChem Compound. U.S. National Library of Medicine: National Center for Biotechnology Information. 3 August 2019. Retrieved 8 August 2019.
(4-hydroxyphenyl)acetaldehyde is an alpha-CH2-containing aldehyde and a member of phenylacetaldehydes. It has a role as a human metabolite, an Escherichia coli metabolite and a mouse metabolite.
- Torrens-Spence, Michael P.; Gillaspy, Glenda; Zhao, Bingyu; Harich, Kim; White, Robert H.; Li, Jianyong (11 February 2012). "Biochemical evaluation of a parsley tyrosine decarboxylase results in a novel 4-hydroxyphenylacetaldehyde synthase enzyme". Biochemical and Biophysical Research Communications. 418 (2): 211–216. doi:10.1016/j.bbrc.2011.12.124. PMID 22266321.
- "4-Hydroxyphenylacetaldehyde". Human Metabolome Database – Version 4.0. University of Alberta. 23 July 2019. Retrieved 8 August 2019.
- Elovaara H, Huusko T, Maksimow M, Elima K, Yegutkin GG, Skurnik M, Dobrindt U, Siitonen A, McPherson MJ, Salmi M, Jalkanen S (2015). "Primary Amine Oxidase of Escherichia coli Is a Metabolic Enzyme that Can Use a Human Leukocyte Molecule as a Substrate". PLOS ONE. 10 (11): e0142367. Bibcode:2015PLoSO..1042367E. doi:10.1371/journal.pone.0142367. PMC 4640556. PMID 26556595.
- Universal protein resource accession number P80668 at UniProt.
- Samanani N, Liscombe DK, Facchini PJ (2004). "Molecular cloning and characterization of norcoclaurine synthase, an enzyme catalyzing the first committed step in benzylisoquinoline alkaloid biosynthesis". Plant Journal. 40 (2): 302–313. doi:10.1111/j.1365-313X.2004.02210.x. PMID 15447655.