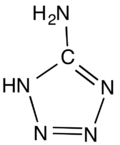
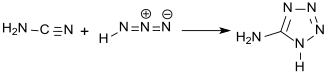5-Aminotetrazole
5-Aminotetrazole is an organic compound with the formula HN4CNH2. It is a white solid that can be obtained both in anhydrous and hydrated forms.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1H-1,2,3,4-Tetrazol-5-amine | |
| Other names
5-ATZ | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.022.348 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CH3N5 | |
| Molar mass | 85.070 g·mol−1 |
| Appearance | White solid |
| Density | 1.502 g/cm3 |
| Melting point | 201–205 °C (394–401 °F; 474–478 K) |
| Hazards | |
| GHS labelling: | |
  | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The molecule is planar.[1] The hydrogen bonding pattern in the hydrate supports the assignment of NH being adjacent to carbon in the ring.[2]
Preparation
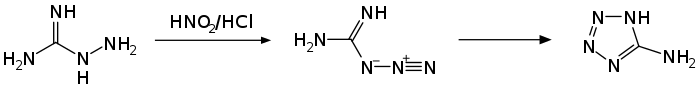
A synthesis of 5-aminotetrazole through the action of nitrous acid on aminoguanidine was reported by Johannes Thiele in 1892.[3]
The exact structure of the compound was not known at the time, although it was known to crystallize as a monohydrate. The correct structural formula was published in 1901 by Arthur Hantzsch, who obtained it from the reaction between cyanamide and hydrazoic acid.[4]
To avoid direct handling of the problematic hydrazoic acid, a mixture of sodium azide and hydrochloric acid has been used to give the monohydrate at 73% yield.[5]
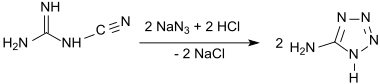
In a more efficient and controllable one-pot synthesis, cyanamide is treated with hydrazine hydrochloride to give aminoguanidine hydrochloride, which is then diazotized as in Thiele's original process. Addition of ammonia or sodium hydroxide followed by heat-induced cyclization gives the anhydrous product in 74% yield.[6][7]
Structure
The structure of 5-aminotetrazole has been determined several times by X-ray crystallography, both as the anhydrous[8] and monohydrated forms.[9] The structures are very similar, consisting of a planar molecule, including the amino group.
Uses
5-Aminotetrazole has found applications in heterocyclic chemistry, particularly as a synthon for some multicomponent reactions.[10]
The N-4 is basic as indicated by its binding to metal halides, such as the coordination complex [CoCl2(aminotetrazole)4.[11]
The compound has a particularly high nitrogen content of 80%. Partly for this reason, the compound is prone to decomposition to nitrogen gas (N2). It has been widely investigated for gas-generating systems, such as airbags and blowing agents.[12]
References
- Hiroshi Fujihisa, Kazumasa Honda, Shigeaki Obata, Hiroshi Yamawaki, Satoshi Takeya, Yoshito Gotoha, Takehiro Matsunaga "Crystal structure of anhydrous 5-aminotetrazole and its high-pressure behavior" CrystEngComm, 2011, volume 13, pp. 99-102. doi:10.1039/C0CE00278J
- D. D. Bray and J. G. White "Refinement of the structure of 5-aminotetrazole monohydrate" Acta Crystallogr. (1979). B35, pp. 3089-3091.doi:10.1107/S0567740879011493
- Thiele, Johannes (1892-01-01). "Ueber Nitro- und Amidoguanidin". Justus Liebigs Annalen der Chemie. 270 (1‐2): 1–63. doi:10.1002/jlac.18922700102. ISSN 0075-4617.
- Hantzsch, A.; Vagt, A. (1901-01-01). "Ueber das sogenannte Diazoguanidin". Justus Liebigs Annalen der Chemie. 314 (3): 339–369. doi:10.1002/jlac.19013140307. ISSN 0075-4617.
- MIHINA, JOSEPH S.; HERBST, ROBERT M. (1950-09-01). "The Reaction of Nitriles with Hydrazoic Acid: Synthesis of Monosubstituted Tetrazoles". The Journal of Organic Chemistry. 15 (5): 1082–1092. doi:10.1021/jo01151a027. ISSN 0022-3263.
- US 5424449, Rothgery, Eugene F. & Knollmueller, Karl O., "Process for the preparation of 5-aminotetrazole", published 1995-06-13, issued 1995-06-13
- US 5594146, Murotani, Masahiro; Mura, Hajime & Takeda, Makoto et al., "Process for producing 5-aminotetrazole", published 1997-01-14, issued 1997-01-14
- Fujihisa, Hiroshi; Honda, Kazumasa; Obata, Shigeaki; Yamawaki, Hiroshi; Takeya, Satoshi; Gotoh, Yoshito; Matsunaga, Takehiro (2011). "Crystal structure of anhydrous 5-aminotetrazole and its high-pressure behavior". CrystEngComm. 13: 99–102. doi:10.1039/c0ce00278j.
- Bray, D. D.; White, J. G. (1979). "Refinement of the structure of 5-aminotetrazole monohydrate". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 35 (12): 3089–3091. doi:10.1107/S0567740879011493.
- Dolzhenko, A. V. (2017). "5-Aminotetrazole as a Building Block for Multicomponent Reactions (Review)". Heterocycles. 94 (10): 1819–1846. doi:10.3987/rev-17-867.
- Zhao, Fang-Hua; Che, Yun-Xia; Zheng, Ji-Min; Grandjean, Fernande; Long, Gary J. (2012). "Two Acentric Mononuclear Molecular Complexes with Unusual Magnetic and Ferroelectric Properties". Inorganic Chemistry. 51 (8): 4862–4868. doi:10.1021/ic300394c. PMID 22480292.
- Lesnikovich, A. I.; Ivashkevich, O. A.; Levchik, S. V.; Balabanovich, A. I.; Gaponik, P. N.; Kulak, A. A. "Thermal decomposition of aminotetrazoles" Thermochimica Acta 2002, vol. 388, pp. 233-251. doi:10.1016/S0040-6031(02)00027-8