Phosphoribosylamine
Phosphoribosylamine (PRA) is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA.[1][2][3] The vitamins thiamine[4] and cobalamin[5] also contain fragments derived from PRA.[6]
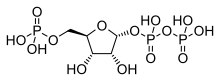 Phosphoribosyl pyrophosphate (PRPP)
Phosphoribosyl pyrophosphate (PRPP)
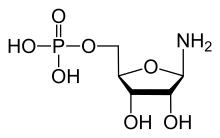 | |
| Names | |
|---|---|
| Other names
PRA | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| MeSH | Phosphoribosylamine |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H12NO7P | |
| Molar mass | 229.125 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
It is the product of the enzyme amidophosphoribosyltransferase which attaches ammonia from glutamine to phosphoribosyl pyrophosphate (PRPP) at its anomeric carbon:[2]
- PRPP + glutamine → PRA + glutamate + PPi
The biosynthesis pathway next combines PRA with glycine in a process driven by ATP giving glycineamide ribonucleotide (GAR). The enzyme phosphoribosylamine—glycine ligase catalyses the reaction forming an amide bond:[7]
- PRA + glycine + ATP → GAR + ADP + Pi
References
- R. Caspi (2009-01-13). "Pathway: 5-aminoimidazole ribonucleotide biosynthesis I". MetaCyc Metabolic Pathway Database. Retrieved 2022-02-02.
- Zhang, Y.; Morar, M.; Ealick, S.E. (2008). "Structural biology of the purine biosynthetic pathway". Cellular and Molecular Life Sciences. 65: 3699–3724. doi:10.1007/s00018-008-8295-8. PMC 2596281. PMID 18712276.
- Gupta, Rani; Gupta, Namita (2021). "Nucleotide Biosynthesis and Regulation". Fundamentals of Bacterial Physiology and Metabolism. pp. 525–554. doi:10.1007/978-981-16-0723-3_19. ISBN 978-981-16-0722-6. S2CID 234897784.
- Chatterjee, Abhishek; Hazra, Amrita B.; Abdelwahed, Sameh; Hilmey, David G.; Begley, Tadhg P. (2010). "A "Radical Dance" in Thiamin Biosynthesis: Mechanistic Analysis of the Bacterial Hydroxymethylpyrimidine Phosphate Synthase". Angewandte Chemie International Edition. 49 (46): 8653–8656. doi:10.1002/anie.201003419. PMC 3147014. PMID 20886485.
- R. Caspi (2019-09-23). "Pathway: 5-hydroxybenzimidazole biosynthesis (anaerobic)". MetaCyc Metabolic Pathway Database. Retrieved 2022-02-10.
- Mehta, Angad P.; Abdelwahed, Sameh H.; Fenwick, Michael K.; Hazra, Amrita B.; Taga, Michiko E.; Zhang, Yang; Ealick, Steven E.; Begley, Tadhg P. (2015). "Anaerobic 5-Hydroxybenzimidazole Formation from Aminoimidazole Ribotide: An Unanticipated Intersection of Thiamin and Vitamin B12 Biosynthesis". Journal of the American Chemical Society. 137 (33): 10444–10447. doi:10.1021/jacs.5b03576. PMC 4753784. PMID 26237670.
- Welin, Martin; Grossmann, Jörg Günter; Flodin, Susanne; Nyman, Tomas; Stenmark, Pål; Trésaugues, Lionel; Kotenyova, Tetyana; Johansson, Ida; Nordlund, Pär; Lehtiö, Lari (2010). "Structural studies of tri-functional human GART". Nucleic Acids Research. 38 (20): 7308–7319. doi:10.1093/nar/gkq595. PMC 2978367. PMID 20631005.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.