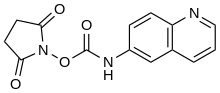
6-Aminoquinolyl-N-hydroxysuccinimidyl carbamate
6-Aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) is a fluorogenic, amine labeling dye that is not fluorescent itself, but covalently reacts with secondary amines to form a fluorescently labeled product.[2] It has a fluorescence excitation wavelength of 250 nm (UV-C), and emission wavelength of 395 nm (deep violet, near UV).[3]
 | |
| Names | |
|---|---|
| IUPAC name
(2,5-dioxopyrrolidin-1-yl) N-quinolin-6-ylcarbamate | |
| Other names
6-AQC, AQC | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| C14H11N3O4 | |
| Molar mass | 285.259 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
See also
References
- "2,5-Dioxopyrrolidin-1-yl quinolin-6-ylcarbamate". PubChem. National Library of Medicine. Retrieved 11 March 2023.
- Stoyanov, Alexander V.; Ahmadzadeh, Hossein; Krylov, Sergey N. (2002-11-25). "Heterogeneity of protein labeling with a fluorogenic reagent, 3-(2-furoyl)quinoline-2-carboxaldehyde". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 780 (2): 283–287. doi:10.1016/s1570-0232(02)00535-4. ISSN 1570-0232. PMID 12401353.
- Cohen, S.A.; Michaud, D.P. (1993). "Synthesis of a Fluorescent Derivatizing Reagent, 6-Aminoquinolyl-N-Hydroxysuccinimidyl Carbamate, and Its Application for the Analysis of Hydrolysate Amino Acids via High-Performance Liquid Chromatography". Analytical Biochemistry. Elsevier BV. 211 (2): 279–287. doi:10.1006/abio.1993.1270. ISSN 0003-2697.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.