AAMP (gene)
Angio-associated, migratory cell protein, also known as AAMP, is a protein which in humans is encoded by the AAMP gene.[5] This protein has been conserved in evolution and is so common to many mammals.[6] and it also has a yeast homolog which is the protein YCR072c.[7][8]
| AAMP | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | AAMP, angio associated migratory cell protein | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 603488 MGI: 107809 HomoloGene: 846 GeneCards: AAMP | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Localisation
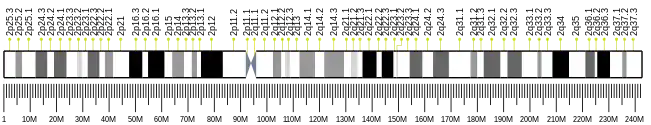
The gene is located on the second human chromosome, near the end of the chromosome's arm (2q35), between the codons 85-87 and 1387–1389. It contains 6042 bp and 11 exons[6] When transcribed, it gives a 1859 bp mRNA. .[6] The vascular endothelial growth factor is a promoting factor of the protein synthesis and localisation in the different parts of the cells.[9] The protein's expression is higher in the intracellular than in the extracellular space.[7]
Function
The gene product is an immunoglobulin-type protein of 434 amino acids and 49 kDa.[6] It is found to be expressed strongly in the cytosol of endothelial cells, cytotrophoblasts, and poorly differentiated colon adenocarcinoma cells found in lymphatics and has been observed at the luminal edges of endometrial cells and in the extracellular environment of vascular-associated mesenchymal cells.[6]
The protein contains a WD40 domain which permits multi-proteins complexes formation [6] and a heparin-binding domain which mediates heparin-sensitive cell adhesion.[5] AAMP helps to regulate vascular endothelial cell migration regulation and angiogenesis, with other signaling pathway like RhoA/Rho-kinase signaling.[9] A malfunction can therefore lead to different diseases (see Associated diseases). For example, in the smooth muscle cells, if AAMP is overexpressed, it activates RhoA, which activates Rho-kinase (this one generates GTP) and it finally leads to increased smooth muscle cell migration and division, causing atherosclerosis and restenosis.[10]
Associated diseases
Note : In all these diseases[6] we can observe the expression of the AAMP gene. This one can either remain stable, increase or decrease depending on the disease.
List of the diseases : gastrointestinal stromal tumor (GIST) (for this disease and the ductal carcinomas, the expression levels are to correlate with necrosis in situ[11]), myeloid leukemia (chronic (CML) and acute (AML) forms), lymphoma, breast cancer, glial brain tumors, colon neoplasia, epidermoid carcinoma, cervical cancer, ovarian cancer, papillary thyroid cancer, pulmonary cancer, atherosclerosis, restenosis.
References
- GRCh38: Ensembl release 89: ENSG00000127837 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000006299 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: AAMP angio-associated, migratory cell protein".
- Beckner ME (March 2012). "AAMP (angio-associated, migratory cell protein)". Atlas of Genetics and Cytogenetics in Oncology and Haematology. 16 (2): 111–114. doi:10.4267/2042/46939.
- Beckner ME, Peterson VA, Moul DE (1999). "Angio-associated migratory cell protein is expressed as an extracellular protein by blood-vessel-associated mesenchymal cells". Microvascular Research. 57 (3): 347–52. doi:10.1006/mvre.1999.2144. PMID 10329261.
- Beckner ME, Liotta LA (July 1996). "AAMP, a conserved protein with immunoglobulin and WD40 domains, regulates endothelial tube formation in vitro". Laboratory Investigation; A Journal of Technical Methods and Pathology. 75 (1): 97–107. PMID 8683944.
- Hu J, Qiu J, Zheng Y, Zhang T, Yin T, Xie X, Wang G (May 2016). "AAMP Regulates Endothelial Cell Migration and Angiogenesis Through RhoA/Rho Kinase Signaling". Annals of Biomedical Engineering. 44 (5): 1462–74. doi:10.1007/s10439-015-1442-0. PMID 26350504. S2CID 52810718.
- Holvoet P, Sinnaeve P (July 2008). "Angio-associated migratory cell protein and smooth muscle cell migration in development of restenosis and atherosclerosis". Journal of the American College of Cardiology. 52 (4): 312–4. doi:10.1016/j.jacc.2008.04.024. PMID 18634988.
- Bielig H, Zurek B, Kutsch A, Menning M, Philpott DJ, Sansonetti PJ, Kufer TA (August 2009). "A function for AAMP in Nod2-mediated NF-kappaB activation". Molecular Immunology. 46 (13): 2647–54. doi:10.1016/j.molimm.2009.04.022. PMID 19535145.
External links
- Human AAMP genome location and AAMP gene details page in the UCSC Genome Browser.
Further reading
- Beckner ME, Krutzsch HC, Stracke ML, Williams ST, Gallardo JA, Liotta LA (May 1995). "Identification of a new immunoglobulin superfamily protein expressed in blood vessels with a heparin-binding consensus sequence". Cancer Research. 55 (10): 2140–9. PMID 7743515.
- Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Beckner ME, Krutzsch HC, Klipstein S, Williams ST, Maguire JE, Doval M, Liotta LA (June 1996). "AAMP, a newly identified protein, shares a common epitope with alpha-actinin and a fast skeletal muscle fiber protein". Experimental Cell Research. 225 (2): 306–14. doi:10.1006/excr.1996.0181. PMID 8660919.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (October 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Beckner ME, Jagannathan S, Peterson VA (May 2002). "Extracellular angio-associated migratory cell protein plays a positive role in angiogenesis and is regulated by astrocytes in coculture". Microvascular Research. 63 (3): 259–69. doi:10.1006/mvre.2001.2384. PMID 11969303.
- Brandenberger R, Wei H, Zhang S, Lei S, Murage J, Fisk GJ, Li Y, Xu C, Fang R, Guegler K, Rao MS, Mandalam R, Lebkowski J, Stanton LW (June 2004). "Transcriptome characterization elucidates signaling networks that control human ES cell growth and differentiation". Nature Biotechnology. 22 (6): 707–16. doi:10.1038/nbt971. PMID 15146197. S2CID 27764390.
- Kimura K, Wakamatsu A, Suzuki Y, Ota T, Nishikawa T, Yamashita R, Yamamoto J, Sekine M, Tsuritani K, Wakaguri H, Ishii S, Sugiyama T, Saito K, Isono Y, Irie R, Kushida N, Yoneyama T, Otsuka R, Kanda K, Yokoi T, Kondo H, Wagatsuma M, Murakawa K, Ishida S, Ishibashi T, Takahashi-Fujii A, Tanase T, Nagai K, Kikuchi H, Nakai K, Isogai T, Sugano S (January 2006). "Diversification of transcriptional modulation: large-scale identification and characterization of putative alternative promoters of human genes". Genome Research. 16 (1): 55–65. doi:10.1101/gr.4039406. PMC 1356129. PMID 16344560.