Methanandamide
Methanandamide (AM-356) is a synthetically created stable chiral analog of anandamide.[1] Its effects have been observed to act on the cannabinoid receptors (specifically on CB1 receptors, which are part of the central nervous system) found in different organisms such as mammals, fish, and certain invertebrates (e.g. Hydra).
 | |
| Names | |
|---|---|
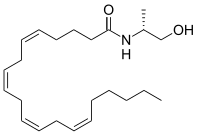
| Preferred IUPAC name
(5Z,8Z,11Z,14Z)-N-[(2R)-1-Hydroxypropan-2-yl]icosa-5,8,11,14-tetraenamide | |
| Other names
AM-356; Arachidonyl-1'-hydroxy-2'-propylamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C23H39NO2 | |
| Molar mass | 361.570 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Abadji V; Lin S; Taha G; Griffin G; Stevenson LA; Pertwee RG; Makriyannis A (1994). "(R)-methanandamide: A chiral novel anandamide possessing higher potency and metabolic stability". Journal of Medicinal Chemistry. 37 (12): 1889–93. doi:10.1021/jm00038a020. PMID 8021930.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.