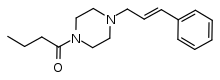
Bucinnazine
Bucinnazine (AP-237, 1-butyryl-4-cinnamylpiperazine) is an opioid analgesic drug that was widely used in China to treat pain in cancer patients as of 1986.[2] It is one of the most potent compounds among a series of piperazine-amides first synthesized and reported in Japan in the 1970s.[3][4][5] Bucinnazine has analgesic potency comparable to that of morphine but with a relatively higher therapeutic index.
 | |
| Clinical data | |
|---|---|
| Other names | AP-237 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C17H24N2O |
| Molar mass | 272.392 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
The drug was initially claimed to be a non-narcotic analgesic. However, subsequent studies have shown bucinnazine and similar acyl piperazines to be potent and selective agonists of μ-opioid receptor (MOR) with relatively low affinity for the δ-opioid receptor and the κ-opioid receptor.[6] In accordance with these studies, results from the intravenous self-administration experiments in rats showed that bucinnazine has a marked reinforcing effect with tolerance and dependence quickly developing.[2] In addition, the morphine antagonist naloxone reverses the effect of bucinnazine and precipitates withdrawal symptoms in bucinnazine treated rats further indicating a mechanism of analgesia mediated via selective agonist activity at μ-opioid receptors.
Derivatives
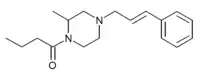
2-methyl-AP-237 has been sold on the grey market as a designer opioid, first identified by a police forensic laboratory in Slovenia in March 2019.[7][8][9]
See also
References
- "2-Methyl-AP-237" (PDF). Office of Diversion Control. U.S Drug Enforcement Administration.
- Qing T, Zhi-Ji C, Wei-Ping W (1986). "Experimental Study on the Dependence-Producing Properties of Qiang Tong Ding (AP-237)". Chin. J. Clin. Pharmacol. (2).
- Nishimura N, Kiuchi M, Kanetake Y, Takahashi T (June 1970). "[Clinical exaluation of a new analgesic agent Ap-237]". Masui. The Japanese Journal of Anesthesiology. 19 (6): 653–656. PMID 4916908.
- Carrano RA, Kimura KK, McCurdy DH (January 1975). "Analgesic and tolerance studies with AP-237, a new analgesic". Archives Internationales de Pharmacodynamie et de Therapie. 213 (1): 41–57. PMID 1156018.
- Carrano RA, Kimura KK, Landes RC, McCurdy DH (January 1975). "General pharmacology of a new analgesic-AP-237". Archives Internationales de Pharmacodynamie et de Therapie. 213 (1): 28–40. PMID 1156016.
- Barlocco D, Cignarella G, Greco G, Novellino E (October 1993). "Computer-aided structure-affinity relationships in a set of piperazine and 3,8-diazabicyclo[3.2.1]octane derivatives binding to the mu-opioid receptor". Journal of Computer-Aided Molecular Design. 7 (5): 557–571. Bibcode:1993JCAMD...7..557B. doi:10.1007/bf00124362. PMID 8294946. S2CID 23360530.
- "Analytical Report 2-Methyl-AP-237" (PDF). Ljubljana, Slovenia: National Forensic Laboratory. 19 March 2019.
- Fogarty MF, Vandeputte MM, Krotulski AJ, Papsun D, Walton SE, Stove CP, Logan BK (June 2022). "Toxicological and pharmacological characterization of novel cinnamylpiperazine synthetic opioids in humans and in vitro including 2-methyl AP-237 and AP-238". Archives of Toxicology. 96 (6): 1701–1710. doi:10.1007/s00204-022-03257-7. PMID 35275255. S2CID 247383361.
- Giorgetti A, Brunetti P, Pelotti S, Auwärter V (October 2022). "Detection of AP-237 and synthetic cannabinoids on an infused letter sent to a German prisoner". Drug Testing and Analysis. 14 (10): 1779–1784. doi:10.1002/dta.3351. PMC 9804899. PMID 35918775.