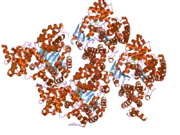APAF1
Apoptotic protease activating factor 1, also known as APAF1, is a human homolog of C. elegans CED-4 gene.[5][6][7]
Function
The protein was identified in the laboratory of Xiaodong Wang as an activator of caspase-3 in the presence of cytochromeC and dATP.[8] This gene encodes a cytoplasmic protein that forms one of the central hubs in the apoptosis regulatory network. This protein contains (from the N terminal) a caspase recruitment domain (CARD), an ATPase domain (NB-ARC), few short helical domains and then several copies of the WD40 repeat domain. Upon binding cytochrome c and dATP, this protein forms an oligomeric apoptosome. The apoptosome binds and cleaves Procaspase-9 protein, releasing its mature, activated form. The precise mechanism for this reaction is still debated though work published by Guy Salvesen suggests that the apoptosome may induce caspase-9 dimerization and subsequent autocatalysis.[9] Activated caspase-9 stimulates the subsequent caspase cascade that commits the cell to apoptosis.
Alternative splicing results in several transcript variants encoding different isoforms.[5]
Structure
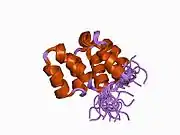
APAF1 contains a CARD domain with a Greek key motif composed of six helices, a Rossman fold nucleotide binding domains, a short helical motif and a winged-helix domain.[10]
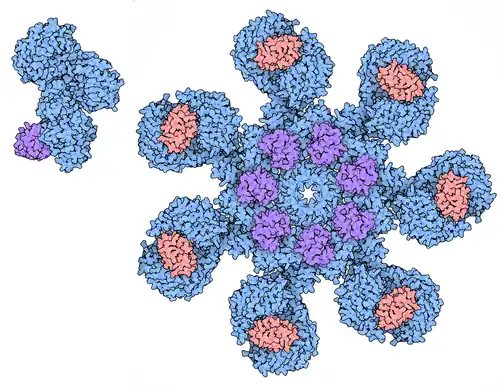
Interactions
APAF1 has been shown to interact with:
References
- GRCh38: Ensembl release 89: ENSG00000120868 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000019979 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: APAF1 apoptotic peptidase activating factor 1".
- Zou H, Henzel WJ, Liu X, Lutschg A, Wang X (Aug 1997). "Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3". Cell. 90 (3): 405–13. doi:10.1016/S0092-8674(00)80501-2. PMID 9267021. S2CID 18105320.
- Kim H, Jung YK, Kwon YK, Park SH (1999). "Assignment of apoptotic protease activating factor-1 gene (APAF1) to human chromosome band 12q23 by fluorescence in situ hybridization". Cytogenetics and Cell Genetics. 87 (3–4): 252–3. doi:10.1159/000015436. PMID 10702682. S2CID 10377371.
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X (Nov 1997). "Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade". Cell. 91 (4): 479–89. doi:10.1016/s0092-8674(00)80434-1. PMID 9390557. S2CID 14321446.
- Pop C, Timmer J, Sperandio S, Salvesen GS (Apr 2006). "The apoptosome activates caspase-9 by dimerization". Molecular Cell. 22 (2): 269–75. doi:10.1016/j.molcel.2006.03.009. PMID 16630894.
- Riedl SJ, Li W, Chao Y, Schwarzenbacher R, Shi Y (Apr 2005). "Structure of the apoptotic protease-activating factor 1 bound to ADP". Nature. 434 (7035): 926–33. Bibcode:2005Natur.434..926R. doi:10.1038/nature03465. PMID 15829969. S2CID 4355459.
- Cho DH, Hong YM, Lee HJ, Woo HN, Pyo JO, Mak TW, Jung YK (Sep 2004). "Induced inhibition of ischemic/hypoxic injury by APIP, a novel Apaf-1-interacting protein". The Journal of Biological Chemistry. 279 (38): 39942–50. doi:10.1074/jbc.M405747200. PMID 15262985.
- Hu Y, Benedict MA, Wu D, Inohara N, Núñez G (Apr 1998). "Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation". Proceedings of the National Academy of Sciences of the United States of America. 95 (8): 4386–91. Bibcode:1998PNAS...95.4386H. doi:10.1073/pnas.95.8.4386. PMC 22498. PMID 9539746.
- Pan G, O'Rourke K, Dixit VM (Mar 1998). "Caspase-9, Bcl-XL, and Apaf-1 form a ternary complex". The Journal of Biological Chemistry. 273 (10): 5841–5. doi:10.1074/jbc.273.10.5841. PMID 9488720.
- Chu ZL, Pio F, Xie Z, Welsh K, Krajewska M, Krajewski S, Godzik A, Reed JC (Mar 2001). "A novel enhancer of the Apaf1 apoptosome involved in cytochrome c-dependent caspase activation and apoptosis". The Journal of Biological Chemistry. 276 (12): 9239–45. doi:10.1074/jbc.M006309200. PMID 11113115.
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X (Nov 1997). "Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade". Cell. 91 (4): 479–89. doi:10.1016/s0092-8674(00)80434-1. PMID 9390557. S2CID 14321446.
- Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES (Aug 2000). "Negative regulation of the Apaf-1 apoptosome by Hsp70". Nature Cell Biology. 2 (8): 476–83. doi:10.1038/35019510. PMID 10934467. S2CID 20374981.
External links
Further reading
- Smith TF, Gaitatzes C, Saxena K, Neer EJ (May 1999). "The WD repeat: a common architecture for diverse functions". Trends in Biochemical Sciences. 24 (5): 181–5. doi:10.1016/S0968-0004(99)01384-5. PMID 10322433.
- van Oirschot JT (Aug 1999). "Diva vaccines that reduce virus transmission". Journal of Biotechnology. 73 (2–3): 195–205. doi:10.1016/S0168-1656(99)00121-2. PMID 10486928.
- Nakajima D, Okazaki N, Yamakawa H, Kikuno R, Ohara O, Nagase T (Jun 2002). "Construction of expression-ready cDNA clones for KIAA genes: manual curation of 330 KIAA cDNA clones". DNA Research. 9 (3): 99–106. CiteSeerX 10.1.1.500.923. doi:10.1093/dnares/9.3.99. PMID 12168954.
- Campioni M, Santini D, Tonini G, Murace R, Dragonetti E, Spugnini EP, Baldi A (Nov 2005). "Role of Apaf-1, a key regulator of apoptosis, in melanoma progression and chemoresistance". Experimental Dermatology. 14 (11): 811–8. doi:10.1111/j.1600-0625.2005.00360.x. PMID 16232302. S2CID 45588573.
- Zou H, Henzel WJ, Liu X, Lutschg A, Wang X (Aug 1997). "Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3". Cell. 90 (3): 405–13. doi:10.1016/S0092-8674(00)80501-2. PMID 9267021. S2CID 18105320.
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X (Nov 1997). "Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade". Cell. 91 (4): 479–89. doi:10.1016/S0092-8674(00)80434-1. PMID 9390557. S2CID 14321446.
- Ishikawa K, Nagase T, Nakajima D, Seki N, Ohira M, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O (Oct 1997). "Prediction of the coding sequences of unidentified human genes. VIII. 78 new cDNA clones from brain which code for large proteins in vitro". DNA Research. 4 (5): 307–13. doi:10.1093/dnares/4.5.307. PMID 9455477.
- Pan G, O'Rourke K, Dixit VM (Mar 1998). "Caspase-9, Bcl-XL, and Apaf-1 form a ternary complex". The Journal of Biological Chemistry. 273 (10): 5841–5. doi:10.1074/jbc.273.10.5841. PMID 9488720.
- Hu Y, Benedict MA, Wu D, Inohara N, Núñez G (Apr 1998). "Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation". Proceedings of the National Academy of Sciences of the United States of America. 95 (8): 4386–91. Bibcode:1998PNAS...95.4386H. doi:10.1073/pnas.95.8.4386. PMC 22498. PMID 9539746.
- Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES (Jun 1998). "Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization". Molecular Cell. 1 (7): 949–57. doi:10.1016/S1097-2765(00)80095-7. PMID 9651578.
- Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P (Sep 1998). "Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development". Cell. 94 (6): 727–37. doi:10.1016/S0092-8674(00)81732-8. PMID 9753320.
- Inohara N, Gourley TS, Carrio R, Muñiz M, Merino J, Garcia I, Koseki T, Hu Y, Chen S, Núñez G (Dec 1998). "Diva, a Bcl-2 homologue that binds directly to Apaf-1 and induces BH3-independent cell death". The Journal of Biological Chemistry. 273 (49): 32479–86. doi:10.1074/jbc.273.49.32479. PMID 9829980.
- Hu Y, Ding L, Spencer DM, Núñez G (Dec 1998). "WD-40 repeat region regulates Apaf-1 self-association and procaspase-9 activation". The Journal of Biological Chemistry. 273 (50): 33489–94. doi:10.1074/jbc.273.50.33489. PMID 9837928.
- Song Q, Kuang Y, Dixit VM, Vincenz C (Jan 1999). "Boo, a novel negative regulator of cell death, interacts with Apaf-1". The EMBO Journal. 18 (1): 167–78. doi:10.1093/emboj/18.1.167. PMC 1171112. PMID 9878060.
- Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ (Jan 1999). "Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner". The Journal of Cell Biology. 144 (2): 281–92. doi:10.1083/jcb.144.2.281. PMC 2132895. PMID 9922454.
- Zou H, Li Y, Liu X, Wang X (Apr 1999). "An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9". The Journal of Biological Chemistry. 274 (17): 11549–56. doi:10.1074/jbc.274.17.11549. PMID 10206961.
- Saleh A, Srinivasula SM, Acharya S, Fishel R, Alnemri ES (Jun 1999). "Cytochrome c and dATP-mediated oligomerization of Apaf-1 is a prerequisite for procaspase-9 activation". The Journal of Biological Chemistry. 274 (25): 17941–5. doi:10.1074/jbc.274.25.17941. PMID 10364241.
- Qin H, Srinivasula SM, Wu G, Fernandes-Alnemri T, Alnemri ES, Shi Y (Jun 1999). "Structural basis of procaspase-9 recruitment by the apoptotic protease-activating factor 1". Nature. 399 (6736): 549–57. Bibcode:1999Natur.399..549Q. doi:10.1038/21124. PMID 10376594. S2CID 4403525.
- Drosopoulos NE, Walsh FS, Doherty P (Jun 1999). "A soluble version of the receptor-like protein tyrosine phosphatase kappa stimulates neurite outgrowth via a Grb2/MEK1-dependent signaling cascade". Molecular and Cellular Neurosciences. 13 (6): 441–9. doi:10.1006/mcne.1999.0758. PMID 10383829. S2CID 35458154.