ATP citrate synthase
ATP citrate synthase (also ATP citrate lyase (ACLY)) is an enzyme that in animals represents an important step in fatty acid biosynthesis.[2] By converting citrate to acetyl-CoA, the enzyme links carbohydrate metabolism, which yields citrate as an intermediate, with fatty acid biosynthesis, which consumes acetyl-CoA.[3] In plants, ATP citrate lyase generates cytosolic acetyl-CoA precursors of thousands of specialized metabolites, including waxes, sterols, and polyketides.[4]
| ATP citrate synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 2.3.3.8 | ||||||||
| CAS no. | 9027-95-6 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Human ATP citrate lyase | |||||||
|---|---|---|---|---|---|---|---|
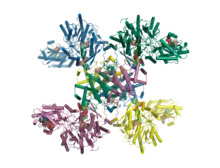 Crystal structure of human ATP citrate lyase in complex with citrate, coenzyme A and Mg.ADP.[1] | |||||||
| Identifiers | |||||||
| Symbol | ACLY | ||||||
| Alt. symbols | ACL | ||||||
| NCBI gene | 47 | ||||||
| HGNC | 115 | ||||||
| OMIM | 108728 | ||||||
| PDB | 3MWE, 3PFF, 5TDE, 5TDF, 5TDM, 5TDZ, 5TE1, 5TEQ, 5TES, 5TET, 6HXH, 6HXK, 6HXL, 6HXM, 6O0H, 6QFB 3MWD, 3MWE, 3PFF, 5TDE, 5TDF, 5TDM, 5TDZ, 5TE1, 5TEQ, 5TES, 5TET, 6HXH, 6HXK, 6HXL, 6HXM, 6O0H, 6QFB | ||||||
| RefSeq | NM_001096 | ||||||
| UniProt | P53396 | ||||||
| Other data | |||||||
| EC number | 2.3.3.8 | ||||||
| Locus | Chr. 17 q21.2 | ||||||
| |||||||
Function
ATP citrate lyase is the primary enzyme responsible for the synthesis of cytosolic acetyl-CoA in many tissues. The enzyme is a tetramer of apparently identical subunits. In animals, the product, acetyl-CoA, is used in several important biosynthetic pathways, including lipogenesis and cholesterogenesis.[5] It is activated by insulin.[6]
In plants, ATP citrate lyase generates acetyl-CoA for cytosolically-synthesized metabolites; Acetyl-CoA is not transported across subcellular membranes of plants. Such metabolites include: elongated fatty acids (used in seed oils, membrane phospholipids, the ceramide moieties of sphingolipids, cuticle, cutin, and suberin); flavonoids; malonic acid; acetylated phenolics, alkaloids, isoprenoids, anthocyanins, and sugars; and, mevalonate-derived isoprenoids (e.g., sesquiterpenes, sterols, brassinosteroids); malonyl and acyl-derivatives (d-amino acids, malonylated flavonoids, acylated, prenylated and malonated proteins).[4] De novo fatty acid biosynthesis in plants occurs in plastids; thus, ATP citrate lyase is not relevant to this pathway.
Reaction
ATP citrate lyase is responsible for catalyzing the conversion of citrate and Coenzyme A (CoA) to acetyl-CoA and oxaloacetate, driven by hydrolysis of ATP.[3] In the presence of ATP and CoA, citrate lyase catalyzes the cleavage of citrate to yield acetyl CoA, oxaloacetate, adenosine diphosphate (ADP), and orthophosphate (Pi):
- citrate + ATP + CoA → oxaloacetate + Acetyl-CoA + ADP + Pi
Structure
The enzyme is composed of two subunits in green plants (including Chlorophyceae, Marchantimorpha, Bryopsida, Pinaceae, monocotyledons, and eudicots), species of fungi, glaucophytes, Chlamydomonas, and prokaryotes.
Animal ACL enzymes are homomeric; a fusion of the ACLA and ACLB genes probably occurred early in the evolutionary history of this kingdom.[4]
The mammalian ATP citrate lyase has a N-terminal citrate-binding domain that adopts a Rossmann fold, followed by a CoA binding domain and CoA-ligase domain and finally a C-terminal citrate synthase domain. The cleft between the CoA binding and citrate synthase domains forms the active site of the enzyme, where both citrate and acetyl-coenzyme A bind.
In 2010, a structure of truncated human ATP citrate lyase was determined using X-ray diffraction to a resolution of 2.10 Å.[3] In 2019, a full length structure of human ACLY in complex with the substrates coenzyme A, citrate and Mg.ADP was determined by X-ray crystallography to a resolution of 3.2 Å.[1] Moreover, in 2019 a full length structure of ACLY in complex with an inhibitor was determined by cryo-EM methods to a resolution of 3.7 Å.[8] Additional structures of heteromeric ACLY-A/B from the green sulfur bacteria Chlorobium limicola and the archaeon Methanosaeta concilii show that the architecture of ACLY is evolutionarily conserved.[1] Full length ACLY structures showed that the tetrameric protein oligomerizes via its C-terminal domain. The C-terminal domain had not been observed in the previously determined truncated crystal structures. The C-terminal region of ACLY assembles in a tetrameric module that is structurally similar to citryl-CoA lyase (CCL) found in deep branching bacteria.[1][9] This CCL module catalyses the cleavage of the citryl-CoA intermediate into the products acetyl-CoA and oxaloacetate. In 2019, cryo-EM structures of human ACLY, alone or bound to substrates or products were reported as well.[10][11] ACLY forms a homotetramer with a rigid citrate synthase homology (CSH) module, flanked by four flexible acetyl-CoA synthetase homology (ASH) domains; CoA is bound at the CSH–ASH interface in mutually exclusive productive or unproductive conformations. The structure of a catalytic mutant of ACLY in the presence of ATP, citrate and CoA substrates reveals a CoA and phosphor-citrate intermediate in the N-terminal domain. Cryo-EM structures of products bound ACLY and substrates bound ACLY were also determined at 3.0 Å and 3.1 Å. An EM structure of mutant E599Q in complex with CoA and phospho-citrate intermediate was determined at resolution of 2.9 Å. Comparison between these structures of apo-ACLY and ligands bound ACLY demonstrated conformational changes on ASH domain (N-terminal domain) when different ligands bind.
Pharmacology
The enzyme's action can be inhibited by the coenzyme A-conjugate of bempedoic acid, a compound which lowers LDL cholesterol in humans.[12] The drug was approved by the Food and Drug Administration in February 2020 for use in the United States.
References
- Verschueren KH, Blanchet C, Felix J, Dansercoer A, De Vos D, Bloch Y, et al. (April 2019). "Structure of ATP citrate lyase and the origin of citrate synthase in the Krebs cycle" (PDF). Nature. 568 (7753): 571–575. Bibcode:2019Natur.568..571V. doi:10.1038/s41586-019-1095-5. PMID 30944476. S2CID 92999924.
- Elshourbagy NA, Near JC, Kmetz PJ, Wells TN, Groot PH, Saxty BA, et al. (March 1992). "Cloning and expression of a human ATP-citrate lyase cDNA". European Journal of Biochemistry. 204 (2): 491–9. doi:10.1111/j.1432-1033.1992.tb16659.x. PMID 1371749.
- Sun T, Hayakawa K, Bateman KS, Fraser ME (August 2010). "Identification of the citrate-binding site of human ATP-citrate lyase using X-ray crystallography". The Journal of Biological Chemistry. 285 (35): 27418–28. doi:10.1074/jbc.M109.078667. PMC 2930740. PMID 20558738.
- Fatland BL, Ke J, Anderson MD, Mentzen WI, Cui LW, Allred CC, et al. (October 2002). "Molecular characterization of a heteromeric ATP-citrate lyase that generates cytosolic acetyl-coenzyme A in Arabidopsis". Plant Physiology. 130 (2): 740–56. doi:10.1104/pp.008110. PMC 166603. PMID 12376641.
- "Entrez Gene: ATP citrate lyase".
- Guay C, Madiraju SR, Aumais A, Joly E, Prentki M (December 2007). "A role for ATP-citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induced insulin secretion". The Journal of Biological Chemistry. 282 (49): 35657–65. doi:10.1074/jbc.M707294200. PMID 17928289.
- ATP+Citrate+Lyase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Wei J, Leit S, Kuai J, Therrien E, Rafi S, Harwood HJ, et al. (April 2019). "An allosteric mechanism for potent inhibition of human ATP-citrate lyase". Nature. 568 (7753): 566–570. Bibcode:2019Natur.568..566W. doi:10.1038/s41586-019-1094-6. PMID 30944472. S2CID 93000843.
- Aoshima M, Ishii M, Igarashi Y (May 2004). "A novel enzyme, citryl-CoA lyase, catalysing the second step of the citrate cleavage reaction in Hydrogenobacter thermophilus TK-6". Molecular Microbiology. 52 (3): 763–70. doi:10.1111/j.1365-2958.2004.04010.x. PMID 15101982. S2CID 32105039.
- Wei X, Schultz K, Bazilevsky GA, Vogt A, Marmorstein R (January 2020). "Molecular basis for acetyl-CoA production by ATP-citrate lyase". Nature Structural & Molecular Biology. 27 (1): 33–41. doi:10.1038/s41594-019-0351-6. PMC 8436250. PMID 31873304.
- Wei X, Schultz K, Bazilevsky GA, Vogt A, Marmorstein R (May 2020). "Author Correction: Molecular basis for acetyl-CoA production by ATP-citrate lyase". Nature Structural & Molecular Biology. 27 (5): 511–513. doi:10.1038/s41594-020-0421-9. PMC 8439269. PMID 32242119.
- Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, et al. (CLEAR Harmony Trial) (March 2019). "Safety and Efficacy of Bempedoic Acid to Reduce LDL Cholesterol". The New England Journal of Medicine. 380 (11): 1022–1032. doi:10.1056/NEJMoa1803917. PMID 30865796.
Further reading
- Lill U, Schreil A, Eggerer H (July 1982). "Isolation of enzymically active fragments formed by limited proteolysis of ATP citrate lyase". European Journal of Biochemistry. 125 (3): 645–50. doi:10.1111/j.1432-1033.1982.tb06731.x. PMID 6749502.
- Srere PA, Lipmann F (1953). "An enzymatic reaction between citrate, adenosine triphosphate and coenzyme A". Journal of the American Chemical Society. 75 (19): 4874. doi:10.1021/ja01115a547.
External links
- ATP Citrate Lyase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.