Ombrabulin
Ombrabulin was an experimental drug candidate discovered by Ajinomoto and further developed by Sanofi-Aventis.[1] Ombrabulin is a combretastatin A-4 derivative that exerts its antitumor effect by disrupting the formation of blood vessels needed for tumor growth.[2][3]
 | |
| Names | |
|---|---|
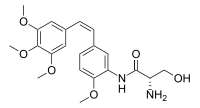
| IUPAC name
N1-{2-Methoxy-5-[(Z)-2-(3,4,5-trimethoxyphenyl)ethen-1-yl]phenyl}-L-serinamide | |
| Systematic IUPAC name
(2S)-2-Amino-3-hydroxy-N-{2-methoxy-5-[(Z)-2-(3,4,5-trimethoxyphenyl)ethen-1-yl]phenyl}propanamide | |
| Other names
AVE-8062; AVE-8062A; AC7700; CS-39-L-Ser.HCl | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H26N2O6 | |
| Molar mass | 402.447 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
It was granted orphan drug status by the European Medicines Agency in April 2011.[4]
In January 2013, Sanofi said it discontinued development of ombrabulin after disappointing results from phase III clinical trials.[5]
References
- "Ombrabulin (AVE8062)". Sanofi-Aventis Oncology.
- Hori, K; Saito, S; Nihei, Y; Suzuki, M; Sato, Y (1999). "Antitumor effects due to irreversible stoppage of tumor tissue blood flow: evaluation of a novel combretastatin A-4 derivative, AC7700". Japanese Journal of Cancer Research. 90 (9): 1026–38. doi:10.1111/j.1349-7006.1999.tb00851.x. PMC 5926172. PMID 10551334.
- Hori, K; Saito, S; Kubota, K (2002). "A novel combretastatin A-4 derivative, AC7700, strongly stanches tumour blood flow and inhibits growth of tumours developing in various tissues and organs". British Journal of Cancer. 86 (10): 1604–14. doi:10.1038/sj.bjc.6600296. PMC 2746587. PMID 12085211.
- "Orphan Designation EU/3/11/853". European Medicines Agency. 15 April 2011.
- "Sanofi has 65 new compounds in development, says R&D chief".
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.